Choosing Fluorescent Reagents for Every Live Cell Application
Scientists monitor dynamic cellular events in real time with live cell imaging and detection techniques. A variety of approaches allow researchers to capture events missed by traditional methods that examine static moments in time, such as qPCR and antibody-based analyses.1-3
Many live cell methods depend on fluorescence detection. In the process of fluorescence, a molecule absorbs light and subsequently emits some of the absorbed energy as photons at a lower energy. A core principle of fluorescence-based methods, from fluorescence microscopy to flow cytometry, is separating excitation light from emitted light with optical filters. This allows scientists to effectively observe and distinguish between physiological processes corresponding to specific fluorescent molecules. Additionally, researchers must select the correct fluorescent indicators for successful live cell imaging and detection. Continual advances in fluorescent probes, dyes, and biosensors improve the power of fluorescence-based approaches, ensuring that such techniques continue to be important research tools in cell biology.1-3
Biosensors for Cell Tracking
Researchers use fluorescent biosensors to efficiently trace living cells in a noninvasive and real-time manner. Tracing live cells over a long period of time provides valuable insight into cellular processes such as the cell cycle and apoptosis. A common example of a widely implemented biosensor in live cell techniques is green fluorescent protein (GFP) and its derivatives. Scientists adapted this fluorescent protein from a naturally-occurring bioluminescence system in jellyfish to achieve long-term cell tracing through genetic cell tagging. However, protein-based biosensors are limited to specific applications, as they typically require expertise in complex transgenic protocols and researchers must verify that biosensor expression is maintained over time.3-5
The Versatility of Fluorescent Probes and Dyes
Probes and dyes that do not rely on genetically-encoded fluorescence offer an alternative to biosensors. Scientists use specific reagents to label subcellular structures like organelles and membranes that are well-defined and carry out specialized functions. Fluorescent staining dyes are available to researchers studying the live cell processes of specific cellular components, including the cell membrane, nucleus, cytoplasm, mitochondria, lysosomes, endoplasmic reticulum (ER), Golgi apparatus, and cytoskeleton proteins. Scientists can also apply organelle dyes as counterstains in live cell imaging, which is useful in functional studies. Additionally, non-organelle, disease-related targets of fluorescent probes and dyes include amyloid plaques, stem cells, neuromuscular junctions, synapses, and tumors.1,3,4,6,7
The Challenge of Choice
Scientists benefit from a variety of biocompatible, inexpensive, and readily available imaging reagents for bioimaging techniques that rely on fluorescence and luminescence. The plethora of instruments and reagents for fluorescence-based methods provides high resolution at the cellular level for almost any application. Modifying reactive groups, substrate moieties, chelating components, and other chemical properties of core fluorophores further expands the possible applications of fluorescent reagents. However, choosing a suitable fluorescent probe, dye, or biosensor to visualize a specific living process can be daunting, given the countless molecules available. Researchers can simplify fluorescent indicator selection by understanding the specific properties and applications of different reagents.4,5,8
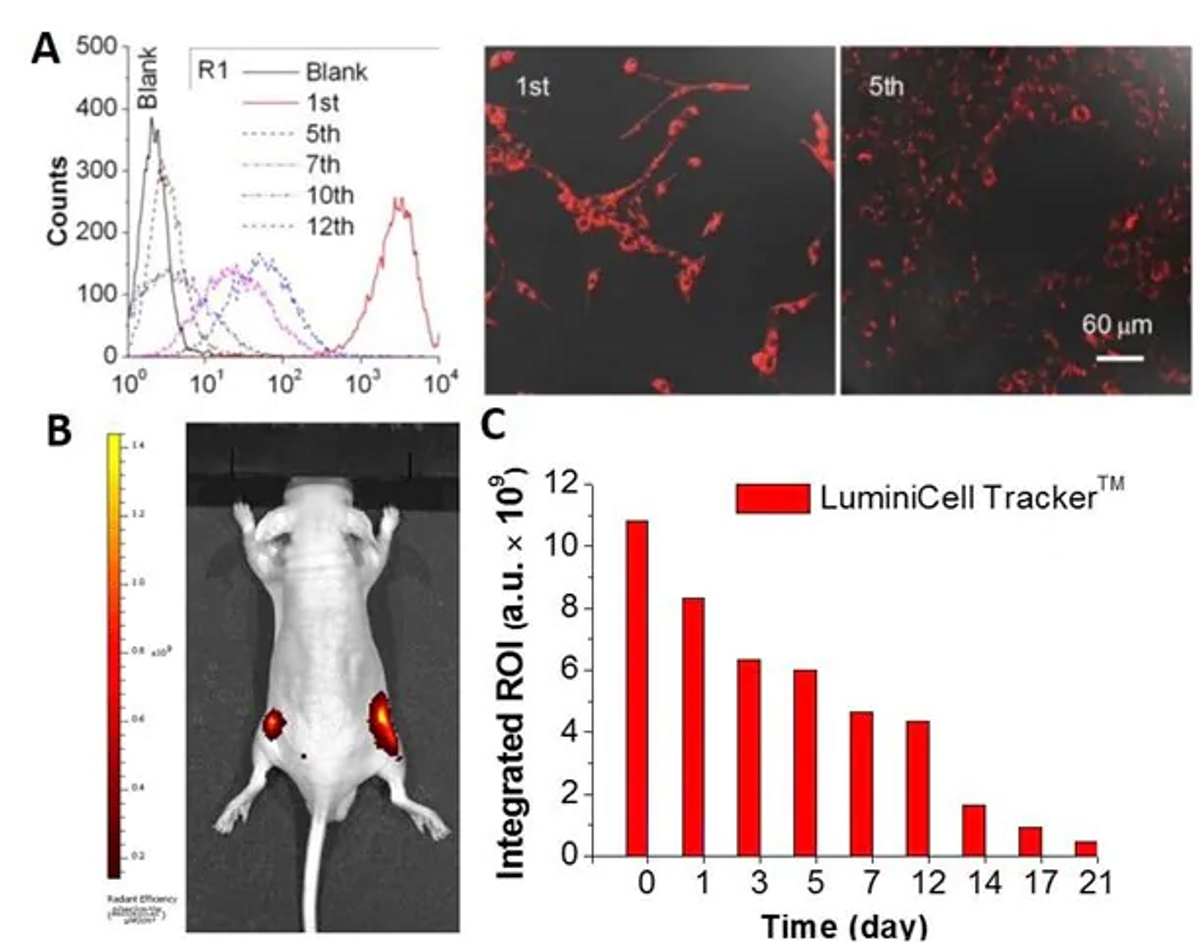
In Vivo Cancer Cell Tracking with LuminiCell Trackers™ A) In vitro tracking of MCF-7 cancer cells with LuminiCell Tracker™-670 Cell Labeling Kit. B) Representative in vivo fluorescence image of mouse subcutaneously injected with 1 × 106 of MCF-7 cells immediately after labelling by 2 nM LuminiCell Tracker™ 670. C) The graph shows the integrated fluorescence intensity at the tumor sites over the total tracking period of 21 days.
MilliporeSigma
Knowing the Options
MilliporeSigma makes choosing live cell imaging reagents easy. Their portfolio of live cell imaging reagents includes novel fluorescent cell labeling technologies, fluorescent lentiviral biosensors, and traditional fluorescent dyes and probes. These reagents are optimal for capturing dynamic cellular events with real-time incubation and imaging systems.3,6
MilliporeSigma offers many fluorescent dyes and markers that are highly specific for diverse organelles. Examples include PKH and CellVue® dyes for long-term, live cell membrane labeling; pre-packaged LentiBrite™ Fluorescence Lentiviral Biosensors that code for proteins involved in autophagy, apoptosis, and cell structure; BioTracker Live Cell dyes for organelle labeling, apoptosis detection, cell viability and health analysis, hypoxia monitoring, ROS tracking, calcium indicator function, and neural and stem cell cultures; and LuminiCellTracker™ fluorescence nanoparticles for long-term cell tracking based on aggregation-induced emission technology.3,4,6,9-11 Improvements in detection technologies and live cell dyes, probes, and fluorescent proteins such as these have advanced researchers’ abilities to visualize and analyze living cells, their intercellular interactions, and subcellular processes with remarkable detail and fidelity.
References
- E.C. Jensen, “Overview of live-cell Imaging: Requirements and methods used,” Anat Rec (Hoboken), 296(1):1-8, 2013.
- M.J. Sanderson et al., “Fluorescence microscopy,” Cold Spring Harb Protoc, 2014(10): 2014.
- “Live Cell Imaging Reagents,” MilliporeSigma, https://www.sigmaaldrich.com/CA/en/products/cell-culture-and-analysis/cell-analysis/live-cell-imaging-reagents, accessed November 7, 2022.
- K. Li et al., “Photostable fluorescent organic dots with aggregation-induced emission (AIE dots) for noninvasive long-term cell tracing,” Sci Rep, 3:1150, 2013.
- V. Baubet et al. “Chimeric green fluorescent protein-aequorin as bioluminescent Ca21 reporters at the single-cell level,” PNAS, 97(13):7260-65, 2000.
- “Imaging Analysis & Live Cell Imaging,” MilliporeSigma, https://www.sigmaaldrich.com/CA/en/applications/cell-culture-and-cell-culture-analysis/cell-analysis/imaging-analysis-and-live-cell-imaging, accessed November 7, 2022.
- “Live Cell Fluorescent Organelle Dyes and Stains,” MilliporeSigma, https://www.sigmaaldrich.com/CA/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/imaging-analysis-and-live-cell-imaging/organelle-dyes-and-stains, accessed November 7, 2022.
- L.D. Lavis, R.T. Raines, “Bright ideas for chemical biology,” ACS Chem Biol, 3(3):142-55, 2008.
- “Live Cell Calcium Indicators,” MilliporeSigma, https://www.sigmaaldrich.com/CA/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/imaging-analysis-and-live-cell-imaging/calcium-indicators, accessed November 7, 2022.
- “Live Cell Imaging of Autophagy using LentiBrite™ Fluorescent Biosensors,” MilliporeSigma, https://www.sigmaaldrich.com/CA/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/imaging-analysis-and-live-cell-imaging/lentibrite-live-cell-lc3-autophagy, accessed November 7, 2022.
- “Long-Term Live Cell Tracking of Cancer and Stem Cells Using a Biocompatible Fluorescent Nanoparticle Based Upon Aggregation Induced Emission (AIE Dot) Nanotechnology,” MilliporeSigma, https://www.sigmaaldrich.com/CA/en/technical-documents/technical-article/cell-culture-and-cell-culture-analysis/imaging-analysis-and-live-cell-imaging/luminicell-live-cell-tracking-nanoparticles, accessed November 7, 2022.