Combined Photodynamic and Photothermal Therapy and Immunotherapy for Cancer Treatment: A Review
Introduction
Cancer has become a major threat to human life health, and its incidence is increasing annually.1 Traditional cancer treatments include surgery, chemotherapy and radiotherapy, which are effective but have serious side effects. Emerging cancer treatment strategies, such as phototherapy, targeted therapy and immunotherapy, have raised hope for cancer patients.2–4 Phototherapy methods include photodynamic therapy (PDT), photothermal therapy (PTT) and photoactive therapy.
PDT is the method of photosensitising drug and laser activation for the treatment of tumour diseases by irradiating tumour sites with a specific wavelength laser; it can activate the photosensitising drug, which is selectively concentrated in the tumour tissue, to trigger a photochemical reaction and destroy tumour tissues.5 However, in the early application of PDT, issues, such as light penetration and tumour hypoxia, limited the further development of PDT; investigators solved this therapeutic challenge by separating photosensitisation events from the delivery of singlet oxygen.6 The new generation of photosensitising drugs in PDT transfers energy to the surrounding oxygen to generate a highly active singlet oxygen; the singlet oxygen energy oxidises with nearby biomacromolecules to induce cytotoxicity, which in turn kills tumour cells.7 Gündüz et al have successfully applied an efficient PDT based on nano-carriers, which are composed of universal spin converter, full, light-harvesting unit and boron-dipyrromethene dyes conjugated with targeting units to fight against cancer.8 In addition, the photosensitisers (PSs) used in PDT have fluorescence properties, and fluorescence-guided imaging has the advantage of better spatial and temporal and safer imaging than traditional imaging methods and can provide safer image guidance for tumour treatment.9 PTT is characterised by excellent anticancer effects, high specificity, low invasiveness and low side effects, and its principle is to utilise the photothermal effect of photothermal conversion agents to obtain energy from visible or near-infrared (NIR) light and convert it into thermal energy, thereby increasing the temperature of the local environment of cancer tissue, which leads to cancer cell death.10 Moreover, photothermal enhancement can accelerate the consumption of glutathione and achieve efficient anti-cancer treatment.11,12 The combination of PTT and immunotherapy has a good therapeutic effect on advanced breast cancer.13 Immunotherapy is a therapeutic approach that enhances anti-tumour immunity by stimulating or modulating the immune system of the body, including oncolytic viruses, immune checkpoint inhibitors, therapeutic antibodies and cancer vaccines, to control and kill tumour cells.14,15 Among these strategies, cytotoxic T cell-associated antigen-4, programmed cell death receptor 1 (PD-1) and programmed cell death ligand 1 (PD-L1) have been approved by the US Food and Drug Administration (FDA) as immune checkpoint inhibitors for solid tumours such as breast cancer.
Great achievements have been made in cancer treatment, from monotherapy in the early stages to combination therapy in recent years. Wang et al used PDT combined with immunotherapy to treat patients with advanced oesophageal cancer with significant results; additional immune checkpoint inhibitors and chemotherapy eliminated the tumour residual tissue, and the patients had a good prognosis, that is, the primary lesion was not only cured, but the metastatic lymph nodes were also significantly reduced.16 Several scholars have also studied the combination of PDT, PTT and immunotherapy in the treatment of tumours and attained excellent efficacy.17 However, at present, a review of the combined application of the above three therapies for tumour treatment is lacking. Therefore, this review mainly describes the effects of these therapies on tumour treatment, their mechanisms, and mediated nanoparticles, and proposes possible directions for further research.
PDT and Its Anticancer Research
PDT
PDT is a clinical approach to treating non-invasive tumours using PSs, oxygen, light and selective photodynamics, and it can be combined with PSs to produce a large amount of reactive oxygen species (ROS) under specific light irradiation wavelength, kill tumour cells and inhibit their growth.18 PDT is widely used in the treatment of solid tumours (such as bladder, oesophageal, skin and breast cancers), vascular, dental and other diseases due to its low toxicity, lack of drug resistance and mild (or no) adverse reactions.19
Mechanism of PDT
Generation of ROS
ROS include a variety of derivatives of molecular oxygen, which is a by-product of normal aerobic metabolism. In recent years, ROS have been considered “toxic” cellular waste. Enhanced ROS levels can indicate irreversible oxidative events that cause permanent damage to macromolecular substances, such as DNA, lipids and proteins, which lead to cell death and/or the potential development of diseases.20 Piskounova et al showed that increased levels of ROS in normal cells increase the chance of gene mutation and promote the transformation of normal cells into tumour cells, that is, ROS can promote the stabilisation of important signalling molecules for tumorigenesis and progression; thus, ROS are not only substances that promote tumour production but also factors that stimulate tumour deterioration.21 Multiple studies have shown that the ROS derivative singlet oxygen can induce tumour cell apoptosis and necrosis.22
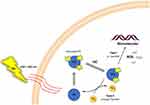
PDT is based on the combination of a PS and strong light. The PS attaches to microbial membranes and binds to microbial surfaces to absorb energy from light, which initiates type I and type II reactions when the PS absorbs photons and is excited by electrons to a singlet state (1PS*) (Figure 1). The excited state PS can undergo intersystem crossing to form a relatively long-lived triplet state (3PS*). In type I reactions, 3PS* can transfer electrons to adjacent biomolecules or directly to oxygen, resulting in the formation of free radicals capable of reacting with O2 to produce O2•–, H2O2 and/or OH•. Alternatively, in type II reactions, the energy of excited state PS can be directly transferred to molecular oxygen to form singlet oxygen 1O2, which has a very short lifetime and reacts with biomolecules only in the micrometre range of its site of generation.23,24
 |
Figure 1 Mechanism of photodynamic therapy. Type I and type II reactions are initiated when the photosensitizer (PS) absorbs a photon and is electronically excited to the singlet state (1PS*). The excited PS may undergo intersystem crossing (ISC) to form a relatively long-lived triplet state (3PS*). In the type I reaction, the 3PS* can transfer an electron to neighboring biomolecules, or directly to oxygen, resulting in radical formation capable of reaction with O2 to produce O2•–, H2O2 or/and OH•. Alternatively, in a type II reaction, energy of the excited PS may be directly transferred to molecular oxygen, to form singlet oxygen, 1O2. Note: Reprinted with permission from Elsevier, 1872(2), Donohoe C, Senge MO, Arnaut LG, Gomes-da-Silva LC. Cell death in photodynamic therapy: From oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer. Copyright 2019, with permission from Elsevier.24 |
Most of the current PDTs are highly dependent on the concentration of O2 within the tumour with great consumption, but a certain degree of hypoxia exists inside the tumour microenvironment (TME); to alleviate the problem of hypoxia, Nanjing Institute of Technology and the National Institutes of health collaborated to prepare a nanosystem for the sustained release of 1O2 for tumour PDT under dark and light hypoxic conditions. Firstly, the semiconductor organic compound tetrastyrene-functionalised pyrrolopyrrolidone-tetraphenylethylene (DPPTPE) was synthesised, and it exhibited high 1O2 generation capacity in CH2Cl2 and water; then, the diblock polyethylene glycol (2-pyridone-based amphiphilic diblock polymer, PEG-Py) functionalised with hydroxypyridine was encapsulated. Under light conditions, the pyridone in PEG-Py can capture the 1O2 generated by DPPTPE under laser irradiation and form a stable endoperoxide intermediate to realise chemical storage, whereas in dark and hypoxic TME, the endoperoxide will chemically release 1O2 to realise sustained tumour PDT effect.25
Mechanism of ROS in Apoptosis
A typical feature of tumours is the deregulation of cell regulation. Thus, the induction of apoptosis by a variety of cytotoxic anticancer drugs is one of the most effective methods for tumour treatment. ROS signalling stimulates the activation of mitochondrion-dependent death pathways by activating mitogen-activated protein kinase pathways, leading to upregulation of the expression of pro-apoptotic proteins and downregulation of anti-apoptotic protein levels, followed by mitochondrial membrane permeabilisation and cell death.26 The ROS produced by PDT, in turn, trigger endoplasmic reticulum stress and cause downstream red signalling pathways, which can effectively promote the development of endoplasmic reticulum.27 Deng et al proposed that certain synthetic nanoparticles can selectively accumulate in the endoplasmic reticulum, locally produce ROS, induce endoplasmic reticulum stress, amplify immunogenic cell death and activate immune cells, thereby enhancing the effect of immunotherapy.28 Hou et al showed that ROS damages molecular models associated with endoplasmic reticulum and triggers immunogenic cell death, maturation of dendritic cells and proliferation of CD8+ T cells, releasing tumour necrosis factor (TNF)-α with interferon (IFN)-γ.29
According to Josephs et al,30 the antitumor activity of TNF-α can be mediated by a variety of mechanisms, including (1) apoptosis by binding to tumour cell surface receptors; (2) activation of macrophages and natural killer (NK) cells by blocking regulatory T cells (Treg cells) as immunosuppressive factor;31,32 (3) inducement of the collapse of tumour microvascular system through endothelial cell regulation and disruption of neoangiogenesis, including damage to the tumour vasculature;33 (4) promotion of tumour-associated macrophages to the M1 anti-tumour stage; (5) attraction and stimulation of activation sites of anti-tumour immune response by neutrophils and monocytes;34 (6) downregulation of interleukin (IL)-13 expression by eosinophils and inhibition of tumour-induced differentiation of monocytes and immunosuppressive phenotypes.35
IFN- γ can regulate the activities of CD4+, CD8+, NK and NK T cells, which are reactive to tumour antigens or exert their anti-tumour effect by inducing apoptosis.36 A recent study revealed the role of low levels of IFN-γ in inducing tumour progression and clarified different signalling pathways activated by IFN-γ in a dose-dependent manner to accelerate cancer progression in patients with multiple cancer types, whereas high levels of IFN-γ can induce apoptosis in non-small-cell lung cancer cells by activating the Janus kinase 1–signal transducer and activator of transcription 1–caspase pathway.37,38
Effect of PDT on Vascular Endothelial Growth Factor (VEGF)
PDT promotes hypoxia in tumour cells and leads to overexpression of angiogenic markers, such as VEGF;39 VEGF-A is a key regulator of angiogenesis and one of the target genes of hypoxia inducible factor (HIF); HIF-1α is a key mediator of oxygen homeostasis under hypoxic conditions, and the body can induce oxygen damage after PDT, which in turn activates angiogenic molecules and affects therapeutic efficacy.40–42 Dai et al showed that VEGF plays a role in tumour vessel growth not by increasing the number of vessels but by other mechanisms, such as increased vascular permeability, which make it favouring to the diffusion of nutrients.43
Multiple studies have shown that PDT combined with anti-VEGF therapy can effectively inhibit tumour growth. Akiko Miki et al’s study showed that PDT combined with anti-VEGF therapy significantly improved the anatomy and visual function of the patient’s eye after it was first used in patients with thick choroid-like neovasculopathy.44 Commonly used anti-VEGF agents include bevacizumab, ranibizumab and others.45
As reported by Jiang et al, P-21-activated kinase 1 (PAK1) is upregulated in PDT and associated with tumour angiogenesis. Activation of PAK1 prevents HIF-1α protein ubiquitin-mediated degradation, and thereafter, HIF-1α accumulation leads to upregulation of VEGF, thereby promoting tumour angiogenesis and can effectively inhibit tumour angiogenesis after PAK1 knockdown, suggesting that PAK1 is a potential novel drug target for inhibiting PDT-induced tumour angiogenesis.46 In addition, Zhao et al showed that a VEGF aptamer with G-quadruplex (G4) structure can deliver phenyl-4-N-methyl-4-pyridyl porphyrin (TMPyP), and the combined administration of daunomycins and TMPyP evidently produced a large amount of ROS, cell cycle arrest and apoptosis and exhibited good cytotoxicity compared with single administration; the dual drug-loaded system exhibited the synergistic effect of chemotherapy/PDT and can significantly inhibit tumour growth.47
PSs
Hematoporphyrin Derivatives (HPDs) (First-Generation PSs)
HPDs are first-generation PSs, and they can produce a certain amount of singlet oxygen after laser irradiation; singlet oxygen is a key factor in killing tumour cells, and its damage mechanism is related to the peroxidation of lipids, cell membranes and nucleic acids, which is still clinically used to treat cervical cancer, oesophageal cancer, colorectal cancer, oral squamous cell carcinoma, etc.48 Several scholars isolated a mixture of porphyrin dimers and oligomer “photoprotein” from HPD, which is also currently one of the commonly used PSs, for clinical treatment.49 Despite its wide application in PDT, several limitations in clinical application, such as low chemical purity, relatively short absorption peak (630 nm), long half-life and high accumulation in the skin, still exist.7 According to the study on He La cell line by Cozzani et al, treatment with liposomal structures containing HPD as active substance has a strong PDT effect.50 Although first-generation PSs have achieved better results in clinical treatment, they also presented unavoidable drawbacks, such as complicated composition and low singlet oxygen production.
Indocyanine Green (ICG) (Second-Generation PSs)
ICG is a NIR dye that has been approved by the FDA for biomedical applications due to its low toxicity, high affinity and unique optical characteristics, including a very strong absorption band (780 nm) and an effective emission band (800–820 nm) in the NIR portion of the spectrum.51 ICG, as a PS, can absorb NIR light and produce ROS or convert into thermal energy for tumour PDT and PTT.52 However, the rapid aggregation of ICG in polar solvents, its poor aqueous solubility, limited tumour accumulation, low bioavailability, low photobleaching and photothermal efficiency, poor stability, rapid clearance in the plasma, short circulation time in vivo and lack of tumour cell targeting specificity are drawbacks that limit its application.10 However, certain studies utilised the advantages of ICG and constructed novel hybrids in combination with other materials for tumour therapy. Liu et al constructed c(RGDfk)-modified glycolipid-like micelles (cRGD-CSOSA) to encapsulate ICG for dual targeting of neovascular endothelial and tumour cells. In vitro, cRGD-CSOSA/ICG inhibited cell proliferation and blocked angiogenesis by NIR irradiation; in vivo, the accumulation of cRGD-CSOSA/ICG in neovascular endothelial and tumour cells increased; by NIR irradiation, the tumour inhibition rate of cRGD-CSOSA/ICG reached 80%, which was significantly higher than that of ICG and CSOSA; histological evaluation showed that tumour blood vessels decreased, and tumour cell apoptosis increased in cRGD-CSOSA/ICG-mediated treatment.53
Supramolecular PSs (Third-Generation PSs)
Based on the host–guest interaction between cationic 4,4-difluoro-boradiazaindacene (BODIPY) derivatives and cucurbit[7]urils (CB[7]), Yuan et al, Tsinghua University, successfully prepared supramolecular PS (BDP2IPh CB[7]) with enhanced ROS generation efficiency and accelerated self-degradation capability; and compared with PS itself (BDP2IPh), BDP2IPh CB[7] exhibited equally excellent PDT efficiency with negligible dark cytotoxicity and better biocompatibility.54 In addition, the supramolecular PS can be degraded by self-generated ROS, presumably for two reasons; one is that CB[7] can enhance the ROS generation capability of BDP2IPh, thus favouring the oxidation-induced degradation of supramolecular PS under light irradiation, which converts it into a small molecule with low cytotoxicity; on the other hand, CB[7] can regulate the activity of BDP2IPh to promote the oxidisation of supramolecular PS.54 In addition, unlike the PS reported by Yuan et al, Li et al55 showed that the tumour pH-responsive supramolecular PS (layered double hydroxide (LDH)-zinc octasulfonate-modified phthalocyanine (ZnPcS8)) was not photoactive under neutral conditions, but it can be effectively activated in a slightly acidic environment (pH = 6.5). LDH-ZnPcS8 was prepared by a simple coprecipitation method based on the electrostatic interaction between negatively charged ZnPcS8 and the cationic layer of LDH. Compared with ZnPcS8 alone, the photodynamic activity of LDH-ZnPcS8 in cancer cells was significantly enhanced in vitro. Fluorescence imaging results of LDH-ZnPcS8 in vivo showed that the nano-compound was activated in tumour tissue and can cause 95.3% growth inhibition of tumour cells, showing a good PDT effect. Moreover, the skin phototoxicity of LDH-ZnPcS8 was very low, and it has great potential to be used as a new PS to activate PDT.55
PSs Based on Nanotechnology
Based on the excellent properties of nanomaterials, numerous scholars have conducted research on the use of nanomaterials to create PSs and have attained certain achievements. Sun et al developed an intelligent pH-controllable and H2O2-responsive nanoplatform with degradable properties.56 The system is based on honeycomb manganese oxide (hMnO2) nanospheres and loaded with photodynamic agent chlorine (Ce6)-sensitised up-conversion nanoparticles (up-conversion nano-phosphors, UCNPs) with a core-shell-shell structure. In this system, the rapid decomposition of hMnO2 nanostructure resulted in the release of Ce6-sensitised UCNPs in an acidic solution containing H2O2. When exposed to laser with wavelength of 808 nm, UCNP emitted visible photons with a high energy, which was absorbed by Ce6 and generated ROS with cytotoxicity, triggering PDT. Similarly, Yang et al used hMnO2 nanoparticles combined with PEG, Ce6 and doxorubicin to obtain H-MnO2-PEG/C&D particles, which triggered a series of anti-tumour reactions.57 According to the research of Sun et al, hMnO2 can also be used as a multi-mode biological imaging contrast agent, such as in magnetic resonance imaging and CT imaging, for imaging-guided diagnosis and treatment and thus has potential value in tumour treatment.56
Anti-Tumour Treatment by PDT
The anti-vascular effect of PDT leads to thrombosis and haemorrhage within tumour vessels, which then leads to tumour death by deprivation of oxygen and nutrients. Another effect of PDT is the uptake of the PS by cancer cells, which then die directly by apoptosis or necrosis; acute inflammation, along with the release of cytokines and proteins in stress-induced PDT tumour response, can lead to an influx of leukocytes, which can contribute to tumour destruction and stimulate the immune system to recognise and destroy cancer cells.58,59 Data showed that if used properly, PDT is an effective alternative treatment option in oncology, including deeper tumors.7,60–62 PDT involves a multistage response, and its effectiveness depends on the induction of cytotoxic transformation.61,62
In a study by Dos Santos et al, breast adenocarcinoma monolayers of human cells (MDA-MB-231 and MCF-7) showed completely different responses to fluorescent dose indicators (cells were irradiated for 6 or 16 min); although MDA-MB-231 cell death was not sensitive to the fluency index, MCF-7 cells showed a significant (threefold) cell sensitivity to shorter irradiation, and furthermore; moreover, those two types of cells were not associated with cell death, and the reduction in intracellular glutathione led to a redox imbalance.63 Most breast cancer patient deaths are associated with cancer cell metastasis. Therefore, inhibition of cancer cell metastasis may provide direction for breast cancer treatment. A study on pyropheophorbide-α methyl ester-mediated PDT (MPPa-PDT) inhibited MCF-7 metastasis in breast cancer cells showed that, MPPa-PDT decreased F-actin expression in MCF-7 cytoskeleton, and after MPPa-PDT, the migration and invasion of MCF-7 cells were significantly reduced.64 These results indicate that MPPa-PDT can effectively inhibit the metastasis of MCF-7 cells and is expected to become one of the therapeutic methods to inhibit the metastasis of cancer cells.
PDT is also an effective treatment modality for all liver cancers and improves patients’ quality of life.65 Liver cancer mainly includes hepatocellular carcinoma (HCC), cholangiocarcinoma and mixed cancer, and it is the second leading cause of cancer death in the world, second only to lung cancer, with a mortality rate of over 95%, especially in the case of HCC.66 Multiple mechanisms are involved in PDT-mediated tumour cell killing of HCC in vitro and in vivo. Shi et al used sinus porphyrin as a PS in the treatment of HCC, including bel-7402 and HepG2 cell lines, and the results suggested that PDT may be caused by mitochondrial damage, which in turn triggered an apoptotic response, such as cytochrome c release into the cytoplasm, leading to caspase protein activation, etc.67
PDT not only promote can apoptosis but also trigger inflammation. Hypericin-mediated PDT can regulate the expression of apoptosis-related genes (such as caspase and cytochrome complex), resulting in the death of HepG2 cells and the significant increase in IL-6, whose expression level is closely related to tumour cell apoptosis and caspase activity.68 Several scholars have also suggested that PDT treatment of HCC is related to immune response. Zhang et al established a disease model and observed that the numbers of CD4+, CD8+ and CD19+ cells increased after injection of the vaccine generated by PDT, and this finding was related to tumour growth inhibition.69
PDT is not only used to assist in the treatment of breast and liver cancers but is also applied to bladder cancer, lung cancer, oesophageal cancer, etc. It is also approved by various countries for several superficial tumours, such as head-and-neck tumours, skin tumours, etc. Compared with traditional surgery, PDT has a significant clinical effect.
PTT and Its Anticancer Research
PTT
PTT is a therapeutic method that involves the injection of materials with a high photothermal conversion efficiency (PCE) into the interior of the human body; the material is aggregated near the tumour tissue using targeted recognition technology; under the irradiation of external light sources, such as NIR, the light energy is converted into heat, increasing the temperature of the tumour region and killing cancer cells.70
PTT is a non-invasive clinical treatment and has been widely concerned because of its advantages, such as low side effects, high specificity and multiple treatments.71 Various types of PTT include inorganic photothermal converters (such as gold nanoplates) and organic photothermal converters (such as polydopamine particles). Although the former has a high conversion rate to NIR light, it is cytotoxic, whereas the latter has good biocompatibility and is an ideal PTT.72
Mechanism of PTT
Physical Mechanisms
In PTT, NIR has good permeability to biological tissues and can penetrate deep tissues of the body to heat tumour cells. The selection of laser wavelength is important in this process. Wu et al compared the remaining light intensities of different wavelengths of laser light after passing through a certain depth of tissue and observed that the laser energy loss in the NIR window with a wavelength of 808 nm was lower, and the remaining energy density after passing through the tissue was stronger.73 With the depth of research, scholars have proposed photothermal transduction agents (PTAs), which are also an important factor in the physical mechanism of PTT; PTAs are enriched in the tumour tissue with carriers and convert light energy into heat under NIR irradiation, producing local hyperthermia and inducing cancer cell necrosis or apoptosis. The process involves the excitation of PTAs by the NIR light source to reach a singlet excited state, after which the transition returns to the ground state; then, the PTAs in the same state collide, convert light energy into kinetic energy and cause the temperature increase of the surrounding tissue, achieving the purpose of clinical treatment.74,75
Immunological Mechanism
Compared with normal tissues, tumour tissues face difficulty in dissipating heat and cannot tolerate high temperatures due to vascular malformation, which easily leads to cell death caused by thermal stimulation. Punkchi’s group at Nanyang Technological University, Singapore, developed an organic semiconducting polymer nanoadjuvant (SPNIIR) for second infrared window (NIR-II) photothermal immunotherapy; after SPNIIR injection and laser irradiation, the local temperature increase in tumours caused tumour tissue ablation and induced immunogenic cell death of tumour cells and released danger associated molecular patterns (DAMPs) and tumour-associated antigens (TAAs); in the process, hyperthermia ablates the thermoresponsive lipid shell, and R848 is released on demand in tumour tissues.76 In the study of Li et al, SPNIIR-mediated NIR-II PTT immunotherapy not only enhanced the inhibitory effect on the growth of primary tumours and metastatic tumours but also effectively inhibited the process of lung metastasis in subcutaneous 4T1 breast cancer mice. Moreover, after tumour tissue ablation, TAAs can trigger inflammatory reactions in tumour regions and promote T lymphocyte expression stimulated by relevant antigens. Meanwhile, during PTT, hyperthermia-increased blood perfusion in tumour regions affects the TME and reduces hypoxic areas, thereby enhancing therapeutic efficacy.77
Pathways of Tumour Apoptosis
Heat shock proteins (HSPs), especially HSP70, are ubiquitous molecular chaperones that promote proper protein folding, and their activity is intensified at elevated temperatures. HSP70 can exert anti apoptotic effects by inhibiting the activation of caspase-3 and blocking the stress-activated kinase pathway.78 In addition, down-regulation of HSP70 can reduce the formation of anti-apoptotic-related protein complexes. Wang et al showed that by inhibiting PTT-induced HSP and attenuating anti-apoptotic signalling, cantharidin (CTD) encapsulated by thermal-sensitive liposomes (TSL) coated with gold nanoparticles (GNPs), namely [email protected], which can exhibit potent PTT effects on A431 cells with a clinically acceptable radiation range (Figure 2).79,80 According to Ali et al, from the relative levels and results of HSP70, the HSP70 level of Huh7.5 cells was 10-fold lower than that of HSC and MCF-7 cells, but the apoptosis of Huh7.5 cells significantly increased after PTT compared with the other two cell lines.78 Previous findings have shown that inhibition of HSP function can disrupt cellular homeostasis and interfere with the integrity of protein interactions, thereby reducing cellular heat resistance and increasing the efficiency of PTT.81 Thus, inhibition of HSP70 is an accepted target in cancer therapy.
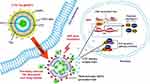 |
Figure 2 Structure of [email protected] and their PTT effects in the tumor cells (International Journal of Nanomedicine 2018 13 2143–2160 “Originally published by and used with permission from Dove Medical Press Ltd”). Abbreviations: BAG-3, Bcl-2-associated athanogene domain 3; BCL-2, B-cell lymphoma-2; CTD, cantharidin; [email protected], CTD-encapsulated TSLs coated with GNPs; GNPs, gold nanoparticles; HSE, heat shock response elements; HSF 1, heat shock transcription factors 1; HSP70, heat shock protein 70; HSR, heat shock response; MCL-1, myeloid cell leukemia-1; NIR, near-infrared; PEG, polyethylene glycol; PTT, plasmonic nanostructure-mediated photothermal therapy; TSL, thermal-sensitive liposome; FITC, fluorescein isothiocyanate. Notes: After their uptake by cancer cells, the PTT effects of the [email protected] are triggered with an NIR laser, and the heat generated induces TSL disruption. Encapsulated CTD and FITC are released into the cytosol, where CTD enhances the PTT effect by suppressing the expression of HSP70 and BAG3 and attenuating anti-apoptotic signaling in the tumor cells. Reprinted with permission from Dove Medical Press Limited. Wang S, Xin J, Zhang L, et al. Cantharidin-encapsulated thermal-sensitive liposomes coated with gold nanoparticles for enhanced photothermal therapy on A431 cells. Int J Nanomedicine. 2018;13:2143-2160. doi: 10.2147/IJN.S156240. Copyright 2018.80 |
PTAs
Inorganic PTAs
Inorganic PTAs include noble metal materials,82 metal sulphur compound materials,10 carbon-based nanomaterials83 and other two-dimensional (2D) materials (eg, black phosphorus, nanosheets, boron nitride and graphitic carbon nitride).84,85 Compared with organic PTAs, inorganic PTAs have higher PCE and better photothermal stability. Noble metal PTAs, including Au, Ag, Pt and PD, can absorb laser energy to excite electrons from the ground state to the excited state and then release energy in the form of heat through nonradiative decay.86 Gold-based PTA has become a research focus because of its advanced synthetic technology, tailorability of adsorption properties, easy manipulation of surface modifications and good stability under biologically relevant conditions.87 At present, gold nanorods are one of the most studied gold-based PTAs, and they have an absorption peak position related to aspect ratio and excellent PCE.88 In the early application process, researchers used cetyltrimethylammonium bromide (CTAB), surfactant sodium oleate and the remodelling process to synthesise Au nanorods with an extremely narrow local surface plasmon resonance band; the results revealed higher monodispersity, smaller width and good photothermal properties. However, given its poor photothermal stability, lack of effective space load and toxicity of CTBA, the later application process, such as in Au nanorings, nanostars and nanoshells, has been gradually improved.89–94
Compared with gold-based PTA, Pd- and Pt-based PTAs have better photothermal stability and certain catalytic properties, and they can maintain structure better under laser irradiation due to their higher melting points.95 Scholars have been attempting to further improve the performance of Pd- or Pt-based PTAs by improving their NIR absorption; Zheng et al synthesised free-standing Pd nanosheets with a thickness of 1.8 nm by increasing their edge length, which can be used to adjust the NIR absorption peak in the range of 826 nm to 1068 nm.95 Unlike gold-based PTA, PD nanosheets can maintain a strong absorbance in the NIR range below 5 nm, and small PD nanosheets not only have good photothermal properties but also exhibit long circulation time, good tumour absorption and renal clearability.96 In addition to good photothermal performance, Pd- or Pt-based PTAs possess several catalytic properties, which can be combined with those of PTA to improve the therapeutic efficacy.97 The limitations of noble metal-based PTAs in PTT have prompted scientists to seek other inorganic materials as PTAs. Graphene has a number of unique characteristics, including large surface-to-volume ratio and excellent electrical and optical properties.84 Given its unique structure and electronic properties, graphene shows plasma properties, which can be used to convert laser energy into heat through plasma photothermal effect.98 Moreover, graphene oxide nanosheets accumulate better in tumours compared with other reported results for carbon nanotubes, and such finding is perhaps related to their 2D structural properties; after systemic administration, no evident toxicity of graphene oxide nanosheets was observed, and tumour cells almost completely regressed after PTT.99 Although the materials described above still have room for the improvement of PCE, the emergence of these carbon-based PTAs has accelerated the development of other graphene analogues, such as MXenes, black phosphorus, etc., which have improved photothermal properties, biodegradability or biocompatibility.100,101 In addition to the above materials, other transition metal-based PTAs, such as quantum dots (Ag2S), metal oxide nanoparticles (copper chalcogenides) and Fe3O4 nanoparticles with controllable size, have been reported by Saeed et al.102–104
Organic Photothermal Conversion Agents
Organic PTAs include NIR responsive small molecules and semiconductor polymer nanoparticles, and they are superior in biodegradability and biocompatibility compared with inorganic PTA.105 Cyanine fuel is composed of two aromatic nitrogen-containing heterocycles interconnected by a polymethionine with tunable length.106 The relatively flexible structure of cyanines differs from those of other small molecules; such a structure allows simple modifications at different locations along the carbon backbone. Cyanine dye Cy3 can be further modified by the addition of a double bond, resulting in a red shift of about 100 nm, or by extending the nitrogen-containing heterocycle to about 20 nm.107 Cyanine molecules, such as ICG, IR780, IR825 and cypate, have excellent photophysical characteristics and become potential candidates for PTT. However, only when ICG is approved by the FDA can it be directly used for diagnosis.108,109 Dyes IR780 and IR825 are mainly designed to have a rigid cyclohexenyl ring in the heptamethionine chain, which can improve the photostability of cyanine dyes, that is, a good photothermal capacity can be achieved after multiple exposures to NIR laser.81 Currently, cyanine-based nanoprobes for PTT have attracted the attention of numerous scholars, but given the fundamental limitations of impaired PCE, photostability and tumour specificity need to be further explored.
Porphyrin is a kind of macromolecular heterocyclic organic compound with conjugated ring structure, which is a multi-heterocyclic conjugated system composed of four pyrrole rings and four carbon atoms. Its derivatives show good photosensitivity, excellent biocompatibility and stability, and compared with normal tissues, they tend to accumulate in tumour sites.110–112 However, the extreme hydrophobicity of porphyrin derivatives, insufficient selectivity to tumour tissues and weak absorption in the NIR region limit their further clinical use.113 In addition, a limited number of scholars have reported porphyrin derivatives with absorption or emission in NIR-II.114,115 Several scholars have observed that perylene diimide (PDI) with a large π–π coupling system has been widely used for tumour therapy due to its excellent photophysical properties and high thermal stability.116 Therefore, Li et al synthesised conjugated porphyrin polymer (P-PPor) with D-A structure via the conjugation of porphyrin (electron donor) and PDI (electron acceptor) and transformed the amphiphilic P-PPor into nanoparticles via a self-assembly method. The results showed that NIR fluorescence quenching, which can be due to the aggregation of molecules in nanostructures, enhanced the photothermal effect; finally, the investigators employed the 4T1 tumour mode to verify the high biocompatibility, excellent photothermal therapeutic activity and low side effects of P-PPor nanoparticles in vitro and in vivo, and their PCE was 66%, which was sufficient for PTT.117 In addition to porphyrins and cyanines, a variety of other organic molecular PTAs exhibit photothermal activity.118 Gao et al designed a heptacyclic B, O-chelating BODIPY structure with strong NIR absorption as a theranostic agent (Boca-BODIPY), and it was further encapsulated into reduced serum albumin (BSA) by self-assembly through simple molecular engineering104 BSA-Boca-BODIPY showed excellent biocompatibility, extraordinary stability and PCE up to 58.7%. The nanoparticles significantly enhanced the photoacoustic contrast in tumour areas, effectively converted laser energy for tumour ablation and killed cancer cells, and no tumour recurrence and PTT-induced toxicity were found after treatment.119 In addition, Liu et al designed and synthesised nanoparticle CR760RGD by covalently linking saffron dye to αVβ3 integrin ligand C (RGDyC) through PEG, which showed strong NIR absorption and high photostability and active tumour targeting and can effectively eliminate tumors.120 Notably, the synthesis of the above two nanoparticles overcomes past limitations, such as hypothermia, etc. However, more relevant studies are needed to further verify the applicability of its clinical PTT.
Antitumor Therapy by PTT
PTT is one of the promising cancer treatments. Chen Hangrong, a researcher of Chinese Academy of Sciences, and Professor Guo of National University of Singapore have developed a new 2D NIR-II biological window nano platform, namely, FePS3 nanosheets based on liquid stripping, which showed an extraordinary Fenton catalytic performance.121 In vitro studies showed that 95% tumour cell inhibition can be induced after half-maximum inhibitory concentration co-incubation with cervical cancer cells for 48 h; tumour eradication was successfully achieved without recurrence after intravenous administration, and this effect was attributed to the high PCE, Fenton catalytic efficiency and their synergistic effect on FePS3 nanosheets; this study demonstrated the excellent biosafety of FePS3 nanosheets in in vivo toxicity tests for up to three months.121 In addition, Qiu et al designed the synthesis of another photothermal conversion agent (ie, PIIGDTS nanoparticles (PD)–folic acid (FA)) with FA using PD with a high PCE of 62.6%; cervical cancer cells were completely eliminated within 18 days after PTT with no toxicity and side effects, exhibiting excellent therapeutic effects for PTT.122
PTT can be used to treat not only cervical cancer but also breast cancer. Triple-negative breast cancer is a highly aggressive malignant disease with high recurrence and metastasis rate, few effective treatment options and poor prognosis. Yang et al constructed PEGylated silver nanotriangles (AENTs) coupled with AS1411 and EpDT3 and evaluated the treatment of breast cancer cells. The results showed that AENTs exhibited excellent targeting characteristics toward breast and cancer stem cells, and its mediated PTT greatly inhibited the migration and invasion of breast cancer cells and the tumour growth and lung metastasis of mice, indicating that AENT-mediated PTT may be one of the effective methods to treat breast cancer.123 Preferred PTT requires effective killing of cancer cells and avoiding adjacent normal tissue damage; therefore, with the help of PTAs with excellent PCE effect, tumours should be irradiated with a low power laser in a short time.10,124 The nanoparticle Fe3O4-Aushell-PEG constructed by Kang et al of the Chinese Army Medical University had a significant therapeutic effect on breast cancer. After three days of PTT treatment, the volume of cancer tissue was significantly reduced to 0.24 times of the original. After six days, except for black scars, almost no tumour was observed.125 All the above studies have shown that PTT has a significant clinical effect on the treatment of breast cancer, and compared with traditional surgery, it has evident characteristics, such as less trauma and fast recovery of patients.
PTT has shown promising clinical effects not only on cervical and breast cancers but also on the treatment of several diseases, such as liver cancer,126 colon cancer127 and rheumatoid arthritis.128
PDT and/or PTT Combined with Immunotherapy
Tumour immunotherapy, an innovative therapy that modulates the immune microenvironment and activates the immune system, relies on auto-immunization to eliminate cancer cells and has the advantage of producing a long-term immunological memory effect without causing damage to normal tissues or cells.129–131 In recent years, with the discovery of tumour immune checkpoints, immunotherapy has emerged as a promising tumour therapy.132 However, immune drugs alone cannot be effective for all patients, and long-term use of drugs can lead to drug resistance or adverse reactions, such as diarrhoea, rash, etc. Therefore, the advantages and disadvantages of PDT, PTT and immunotherapy should be comprehensively considered in the development of appropriate combination therapy for cancer (Table 1).
 |
Table 1 Main Advantages, Disadvantages and Enhancement Strategies for PDT, PTT and Immunotherapy |
Antitumor Treatment of PDT Combined with Immunotherapy
Immune adjuvants refer to all substances that are injected or pre-injected with antigens and can increase the body’s cellular or humoral immune response to antigens. Toll-like receptor (TLR) agonist is one of the pattern recognition receptors expressed by a wide range of immune cells, and it is also an immune adjuvant that can activate the TLR signalling pathway and enhance immune response.135–137 Xu et al used the hydrophobic region between UCNPs and PEG to load the PS Ce6 and the TLR7 agonist R837, and the results showed that TAAs were released from PDT-killed tumour cells and R837, and by upregulating the expression of costimulatory molecules, such as CD80 and CD86, cytokines related to innate and adaptive immunity, such as TNF-α and IL-12, were released to kill tumour cells.138 The combination of PDT and TLR5 agonist flagellin effectively inhibited bilateral melanoma in mice, enhanced the cross presentation of tumour antigens in TME and promoted the infiltration of tumour CD8+ T cells and secretion of IFN-γ in the whole body.139 The above research indicated that PDT immune adjuvant has good research potential and can inhibit tumour metastasis and recurrence.
In the process of PDT combined with immunotherapy to treat tumours, in addition to immune adjuvants, immunosuppressive agents can be employed to suppress tumour immunosuppressive signals and enhance PDT-induced immune responses. Immunosuppressive cells, such as myeloid-derived suppressor cells and regulatory T cells, suppress antitumor immune responses in the TME and promote tumour progression and invasion.140
Several scholars observed that PDT vaccine significantly increased the levels of myeloid-derived suppressor cells and Treg cells, whereas low doses of glycated chitosan and cyclophosphamide decreased the increased levels of immunosuppressive cells.141 These results demonstrate the feasibility of using small molecule inhibitors to attenuate immunosuppressive cells and alter TME inhibition. In addition, tumour tissues can secrete a large amount of VEGF, which promotes the proliferation of immunosuppressive cells and inhibits dendritic cells through the activation of nuclear factor kappa-B pathway. However, excessive VEGF can lead to abnormalities in tumour vascular structure and function and aggravate the hypoxia state of TME, thus affecting the treatment effect of PDT.142,143
As reported by Cramer et al, PDT not only disrupts the original homeostasis of the TME and may support anti-tumour immunity but also induces the production of TAAs and release of DAMPs to promote the maturation of antigen-presenting cells and reprogram the TME to make it more susceptible to immune checkpoint therapy.144 According to Kim et al’s research, local immunogenicity clearance sensitised the blocking reaction of PD-1 or PD-L1 immune checkpoint, reconstructed the TME immunophenotype of cold tumour into hot tumour, led to the aggregation of powerful cytotoxic CD8 T cells in TME, spread systemic anti-tumour immunity to mediate abdominal cavity effect and extended the survival time.145 The study by Yuan et al not only verified the effect of PDT combined with immune checkpoint inhibitors mediated by multifunctional nanoparticles loaded with photosensitizer temoporfin (mTHPC) on colorectal cancer but also illustrated the underlying mechanism by which PDT enhanced the therapeutic efficacy of PD-L1 blockade, that is, hypoxia mediated mainly by PDT can induce HIF-1α pathway, which upregulates PD-L1 expression in colorectal cancer.146
Another combination of PDT and immunotherapy relies on cancer vaccines produced by PDT. Liu et al developed a microfluidic technology to load ICG into liposomes with a high encapsulation efficiency. Under NIR irradiation, the induced endoplasmic reticulum targeting PDT can promote the release of dangerous signal molecules and tumour antigens in vivo, thereby enhancing the immunogenicity in vivo and stimulating a strong anti-tumour immune response.147 Studies have shown that this process can be amplified by dendritic cells, such as PDT dendritic cell vaccines mediated by 5-aminolevulinic acid, which can stimulate the immune response of tumours, leading to a massive increase in IFN-γ+ T cells, which enhances the immune response.148–150 Doix et al pointed out that the vaccination time of PDT dendritic cell vaccine is important because vaccination cannot inhibit tumour growth before radiotherapy, whereas vaccination can significantly inhibit tumour growth around radiotherapy.151
Antitumor Therapy of PTT Combined with Immunotherapy
PTT is a highly effective and non-invasive treatment strategy with minimal damage to surrounding healthy tissues, and thermal ablation can induce immunogenic cell death, promote the release of TAAs, stimulate the maturation of dendritic cells and effectively generate in situ personalised tumour vaccines that activate systemic immune response.152,153 The recurrence and metastasis of numerous kinds of tumours (including breast cancer, melanoma and colorectal cancer) can be effectively inhibited when anticancer drugs plus PTT regimen or tumour hypoxia alleviation therapy is combined with immune checkpoint blocking.154,155 Zhou et al also demonstrated that combination therapy was better than monotherapy. Firstly, they designed HCC-targeting SP94 peptide and SP94-PB-SF-Cy5.5 nanoparticles for HCC-targeting therapy. The results showed that the nanoparticles had extraordinary photothermal effects and achieved the controlled release of sorafenib, thereby eradicating tumours with no local recurrence and minimal toxic side effects. The combination of photothermal and hypoxic alleviating effects produced immune-promoting TME.156
However, using PTT mediated by nanomaterials alone to treat tumours cannot effectively inhibit cancer metastasis due to the need for direct irradiation of light.157 Yan et al pointed out that nanomaterial-mediated PTT in combination with PD-1/PD-L1 blockers can eradicate primary tumours and prevent cancer metastasis in almost all treated mice.158 PD-1/PD-L1 checkpoint is a negative immune regulation mechanism that can be blocked by PD-L1 inhibitor released by heat sensitivity. It can activate the immune system in the treatment of tumour recurrence and metastasis using the synergistic effect of immunogenic death and small-molecule PD-L1 inhibitor.159 Given that the differentiation of tumour-reactive T cells is often inhibited by the transforming growth factor-β (TGF-β) pathway, blocking PD-1/PD-L1 only causes limited immune response. Huang et al inhibited TGF-β pathway by TGF-β inhibitor to drive effector T cells to produce immune response and reduce Treg cell infiltration.160 In addition, by blocking PD-1/PD-L1 immune checkpoint to neutralise the immunosuppressive protective effect of tumour cells, the primary tumour was eliminated, the in situ TAA was exposed, and the immune response to metastasis inhibition was produced, thus realizing the dual remission of PTT-induced immunogenic cell death and immunosuppression.160
In addition, tumour size has an evident effect on the treatment of PTT. In one study, Nam noted that PTT was less effective against large tumours (>100 mm3) compared with small tumours (approximately 50 mm3) due to the limited internal heated and immunosuppressed TME.161 Nam treated tumours with a combination of PTT and neoantigen cancer vaccine and reported that PTT destroyed the TME through heat-induced cellular and molecular damage, sensitising tumours to neoantigen cancer vaccine, which reversed PTT-induced inhibited local immunity and allowed the residual TME to form a morphology favourable for antitumor immunity.162
Antitumor Treatment of PDT and PTT Combined with Immunotherapy
Combination therapy of PDT and PTT is ideal because PDT can increase the sensitivity of tumour cells to PTT by interfering with the TME, disrupting tumour physiology.163 Moreover, the heat generated by PTT can increase blood flow, thereby improving oxygen supply to enhance the therapeutic effect of PDT.164 In addition, the tumour cell debris generated by phototherapy (including PDT and PTT) can act as TAAs and elicit antitumor immune responses to eliminate residual and metastatic cancer cells.138,165 However, the effect of phototherapy is seriously affected by the tissue penetration depth of NIR light, and its anti-tumour immune effect is inadequate to alleviate the immunosuppression of TME.166 Therefore, the combination of photoimmunotherapy and TME regulation may be one of the ideal strategies to improve the antitumor effect. Zhang et al reported that the combination of hyaluronic acid (HA)-BP, which is a nanoparticle modified by PEG HA, PDT and PTT not only significantly inhibited the original tumour but also induced immunogenic cell death and release DAMPs, thereby inducing the maturation of dendritic cells, activating effector cells and strongly arousing anti-tumour immune responses for cancer therapy.167 Hu et al showed that the synergistic treatment of PTT, PDT and chemodynamic therapy not only inhibited the growth of primary tumours but also continuously stimulated the anti-tumour immune response of the system, activated T cells and then effectively inhibit tumour metastasis and growth of distant tumours by interacting with PD-1 or PD-L1 checkpoint blockade (Figure 3).168
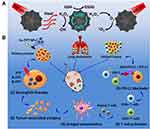 |
Figure 3 (A) Schematic illustration of the Cu-PPT based synergistic PTT, CDT, and PDT therapies. (B) The mechanism of enhanced systemic antitumor immunity induced by the combination of synergistic therapies with PD-1/PD-L1 CBI. Note: Reprinted with permission from Hu C, Cai L, Liu S, et al. Copper-doped nanoscale covalent organic polymer for augmented photo/chemodynamic synergistic therapy and immunotherapy. Bioconjug Chem. 2020;31(6):1661–1670. doi:10.1021/acs.bioconjchem.0c00209.Copyright 2020 American Chemical Society.168 |
In general, PTT and PDT can enhance the immunotherapy response through the following mechanisms:133 (1) immunogenic cell death induced by PTT and PDT can effectively damage tumours through the action of local immune cells; (2) tumour-specific antigens released by the death of immunogenic cells can be used as in-situ vaccines;169 (3) DAMPs enhance the typical weak immunogenicity of natural tumour antigens;170 (4) proinflammatory cytokines up-regulate and promote the activation of immune system. These effects cooperate with immunotherapy to increase the tumour infiltration of cytotoxic CD8+ and effector memory T cells to effectively eliminate the target tumour and residual cancer cells and trigger immune memory to prevent tumour recurrence and provide the possibility of cure.133,171
The above studies illustrated that multimodal therapy of tumours has an improved therapeutic effect and can achieve a high survival rate; Jiang et al’s research proved this viewpoint, reporting that based on the integration of NIR fluorescence, photothermal, photodynamic and immune effects by the nanoplatform, most tumours were excised under intraoperative fluorescence navigation, after which, several microscopic residual tumours were completely ablated by PDT and PT to maximise the killing of tumour cells and tissues with a patient survival rate as high as 90%; importantly, nanoparticle-mediated PDT/PTT plus PD-L1 antibody significantly induced tumour elimination by enhancing immunotherapy.172
The PDT, PTT and immunotherapy mentioned in this review have their own characteristics, whether in vivo or in vitro, and may yield unexpected results with the use of appropriate combination therapy to treat tumours (Table 2).
 |
Table 2 Results of PDT, PTT and Immunotherapy or Their Combination in Treating Tumors in vivo or in vitro |
Summary and Outlook
PDT and PTT have a number of advantages, such as lack of drug resistance, minimal trauma, etc., and they have become several of the effective methods to treat cancer and improve the efficacy of clinical treatment. Especially, PSs or photothermal conversion agents synthesised based on nanotechnology can greatly improve the survival rate of patients by combining phototherapy with immunotherapy. However, the therapeutic effect of phototherapy on tumours largely depends on sufficient laser irradiation dose, and the circulation of nanoparticles in the human body is unclear. Moreover, long-term experimental observation of large samples is lacking. Therefore, the potential long-term effects of phototherapy need to be further and continuously studied. At present, most of the schemes are still under exploration, and a certain gap exists in the actual realisation of extensive clinical treatment. With the progress and development of medical treatment, the multi-mode combined treatment scheme will become a new opportunity and challenge for the new generation of cancer treatments.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Feng R-M, Zong Y-N, Cao S-M, Xu R-H. Current cancer situation in China: good or bad news from the 2018 Global Cancer Statistics? Cancer Commun. 2019;39(1):22. doi:10.1186/s40880-019-0368-6
2. Gulzar A, Wang Z, He F, et al. An 808 nm light-sensitized upconversion nanoplatform for multimodal imaging and efficient cancer therapy. Inorg Chem. 2020;59(7):4909–4923. doi:10.1021/acs.inorgchem.0c00170
3. Zou J, Wang P, Wang Y, et al. Penetration depth tunable BODIPY derivatives for pH triggered enhanced photothermal/photodynamic synergistic therapy. Chem Sci. 2019;10(1):268–276. doi:10.1039/c8sc02443j
4. Cabrita R, Lauss M, Sanna A, et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature. 2020;577(7791):561–565. doi:10.1038/s41586-019-1914-8
5. Mahmoudi K, Garvey KL, Bouras A, et al. 5-aminolevulinic acid photodynamic therapy for the treatment of high-grade gliomas. J Neurooncol. 2019;141(3):595–607. doi:10.1007/s11060-019-03103-4
6. Ayan S, Gunaydin G, Yesilgul-Mehmetcik N, et al. Proof-of-principle for two-stage photodynamic therapy: hypoxia triggered release of singlet oxygen. Chem Commun (Camb). 2020;56(94):14793–14796. doi:10.1039/d0cc06031c
7. Kwiatkowski S, Knap B, Przystupski D, et al. Photodynamic therapy – mechanisms, photosensitizers and combinations. Biomed Pharmacother. 2018;106:1098–1107. doi:10.1016/j.biopha.2018.07.049
8. Gündüz EÖ, Gedik ME, Günaydın G, Okutan E. Amphiphilic Fullerene-BODIPY Photosensitizers for Targeted Photodynamic Therapy. ChemMedChem. 2022;17(6):e202100693. doi:10.1002/cmdc.202100693
9. Sarbadhikary P, George BP, Abrahamse H. Recent advances in photosensitizers as multifunctional theranostic agents for imaging-guided photodynamic therapy of cancer. Theranostics. 2021;11(18):9054–9088. doi:10.7150/thno.62479
10. Liu Y, Bhattarai P, Dai Z, Chen X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem Soc Rev. 2019;48(7):2053–2108. doi:10.1039/c8cs00618k
11. An P, Fan F, Gu D, et al. Photothermal-reinforced and glutathione-triggered in Situ cascaded nanocatalytic therapy. J Control Release. 2020;321:734–743. doi:10.1016/j.jconrel.2020.03.007
12. Kennedy L, Sandhu JK, Harper ME, Cuperlovic-Culf M. Role of glutathione in cancer: from mechanisms to therapies. Biomolecules. 2020;10(10):1429. doi:10.3390/biom10101429
13. Wang M, Rao J, Wang M, et al. Cancer photo-immunotherapy: from bench to bedside. Theranostics. 2021;11(5):2218–2231. doi:10.7150/thno.53056
14. Angell HK, Bruni D, Barrett JC, et al. The immunoscore: colon cancer and beyond. Clin Cancer Res. 2020;26(2):332–339. doi:10.1158/1078-0432.CCR-18-1851
15. Xu D, Liu J, Wang Y, et al. Black Phosphorus Nanosheet with High Thermal Conversion Efficiency for Photodynamic/Photothermal/Immunotherapy. ACS Biomater Sci Eng. 2020;6(9):4940–4948. doi:10.1021/acsbiomaterials.0c00984
16. Wang XY, Maswikiti EP, Zhu JY, et al. Photodynamic therapy combined with immunotherapy for an advanced esophageal cancer with an obstruction post metal stent implantation: a case report and literature review. Photodiagnosis Photodyn Ther. 2022;37:102671. doi:10.1016/j.pdpdt.2021.102671
17. Qiu Q, Li C, Yan X, et al. Photodynamic/ photothermal therapy enhances neutrophil-mediated ibrutinib tumor delivery for potent tumor immunotherapy: more than one plus one? Biomaterials. 2021;269:120652. doi:10.1016/j.biomaterials.2021.120652
18. Gong H, Chao Y, Xiang J, et al. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016;16(4):2512–2521. doi:10.1021/acs.nanolett.6b00068
19. Wan MT, Lin JY. Current evidence and applications of photodynamic therapy in dermatology. Clin Cosmet Investig Dermatol. 2014;7:145–163. doi:10.2147/CCID.S35334
20. Lennicke C, Cochemé HM. Redox metabolism: ROS as specific molecular regulators of cell signaling and function. Mol Cell. 2021;81(18):3691–3707. doi:10.1016/j.molcel.2021.08.018
21. Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527(7577):186–191. doi:10.1038/nature15726
22. Prinz C, Vasyutina E, Lohmann G, et al. Organometallic nucleosides induce non-classical leukemic cell death that is mitochondrial-ROS dependent and facilitated by TCL1-oncogene burden. Mol Cancer. 2015;14:114. doi:10.1186/s12943-015-0378-1
23. Plotino G, Grande NM, Mercade M. Photodynamic therapy in endodontics. Int Endod J. 2019;52(6):760–774. doi:10.1111/iej.13057
24. Donohoe C, Senge MO, Arnaut LG, Gomes-Da-Silva LC. Cell death in photodynamic therapy: from oxidative stress to anti-tumor immunity. Biochim Biophys Acta Rev Cancer. 2019;1872(2):188308. doi:10.1016/j.bbcan.2019.07.003
25. Zou J, Zhu J, Yang Z, et al. A phototheranostic strategy to continuously deliver singlet oxygen in the dark and hypoxic tumor microenvironment. Angew Chem Int Ed Engl. 2020;59(23):8833–8838. doi:10.1002/anie.201914384
26. Hseu Y-C, Lee M-S, Wu C-R, et al. The chalcone flavokawain B induces G2/M cell-cycle arrest and apoptosis in human oral carcinoma HSC-3 cells through the intracellular ROS generation and downregulation of the Akt/p38 MAPK signaling pathway. J Agric Food Chem. 2012;60(9):2385–2397. doi:10.1021/jf205053r
27. Galluzzi L, Kepp O, Kroemer G. Enlightening the impact of immunogenic cell death in photodynamic cancer therapy. EMBO J. 2012;31(5):1055–1057. doi:10.1038/emboj.2012.2
28. Deng H, Zhou Z, Yang W, et al. Endoplasmic reticulum targeting to amplify immunogenic cell death for cancer immunotherapy. Nano Lett. 2020;20(3):1928–1933. doi:10.1021/acs.nanolett.9b05210
29. Hou YJ, Yang XX, Liu RQ, et al. Pathological mechanism of photodynamic therapy and photothermal therapy based on nanoparticles. Int J Nanomedicine. 2020;15:6827–6838. doi:10.2147/IJN.S269321
30. Josephs SF, Ichim TE, Prince SM, et al. Unleashing endogenous TNF-alpha as a cancer immunotherapeutic. J Transl Med. 2018;16(1):242. doi:10.1186/s12967-018-1611-7
31. Nie H, Zheng Y, Li R, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19(3):322–328. doi:10.1038/nm.3085
32. Valencia X, Stephens G, Goldbach-Mansky R, et al. TNF downmodulates the function of human CD4+CD25hi T-regulatory cells. Blood. 2006;108(1):253–261. doi:10.1182/blood-2005-11-4567
33. Hoving S, Seynhaeve AL, van Tiel ST, et al. Early destruction of tumor vasculature in tumor necrosis factor-alpha-based isolated limb perfusion is responsible for tumor response. Anticancer Drugs. 2006;17(8):949–959. doi:10.1097/01.cad.0000224450.54447.b3
34. Qiao Y, Huang X, Nimmagadda S, et al. A robust approach to enhance tumor-selective accumulation of nanoparticles. Oncotarget. 2011;2(1–2):59–68. doi:10.18632/oncotarget.227
35. Kratochvill F, Neale G, Haverkamp JM, et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12(11):1902–1914. doi:10.1016/j.celrep.2015.08.033
36. Rakshit S, Chandrasekar BS, Saha B, et al. Interferon-gamma induced cell death: regulation and contributions of nitric oxide, cJun N-terminal kinase, reactive oxygen species and peroxynitrite. Biochim Biophys Acta. 2014;1843(11):2645–2661. doi:10.1016/j.bbamcr.2014.06.014
37. Alspach E, Lussier DM, Schreiber RD. Interferon γ and its important roles in promoting and inhibiting spontaneous and therapeutic cancer immunity. Cold Spring Harb Perspect Biol. 2019;11(3):a028480. doi:10.1101/cshperspect.a028480
38. Todorović-Raković N. The role of cytokines in the evolution of cancer: IFN-γ paradigm. Cytokine. 2022;151:155442. doi:10.1016/j.cyto.2021.155442
39. Niles DJ. Nanoparticle Delivered VEGF-A siRNA Enhances Photodynamic Therapy for Head and Neck Cancer Treatment. Molecular Therapy. 2016;24(1):106–116. doi:10.1038/mt.2015.169
40. Maxwell PH, Ratcliffe PJ. Oxygen sensors and angiogenesis. Semin Cell Dev Biol. 2002;13(1):29–37. doi:10.1006/scdb.2001.0287
41. Ferrario A, Chantrain CF, Tiehl KV, et al. The matrix metalloproteinase inhibitor prinomastat enhances photodynamic therapy responsiveness in a mouse tumor model. Cancer Res. 2004;64(7):2328–2332. doi:10.1158/0008-5472.can-04-0071
42. Mitra S, Cassar SE, Niles DJ, et al. Photodynamic therapy mediates the oxygen-independent activation of hypoxia-inducible factor 1. Mol Cancer Ther. 2006;5(12):3268–3274. doi:10.1158/1535-7163.MCT-06-0421
43. Dai C, Liang S, Sun B, et al. Anti-VEGF therapy in refractory pituitary adenomas and pituitary carcinomas: a review. Front Oncol. 2021;11:773905. doi:10.3389/fonc.2021.773905
44. Miki A, Kusuhara S, Otsuji T, et al. Photodynamic therapy combined with anti-vascular endothelial growth factor therapy for pachychoroid neovasculopathy. PLoS One. 2021;16(3):e0248760. doi:10.1371/journal.pone.0248760
45. Gao Y, Yu T, Zhang Y, Dang G. Anti-VEGF Monotherapy Versus Photodynamic Therapy and Anti-VEGF Combination Treatment for Neovascular Age-Related Macular Degeneration: a Meta-Analysis. Invest Ophthalmol Vis Sci. 2018;59(10):4307–4317. doi:10.1167/iovs.17-23747
46. Jiang S, Gao Y, Yu QH, et al. P-21-activated kinase 1 contributes to tumor angiogenesis upon photodynamic therapy via the HIF-1α/VEGF pathway. Biochem Biophys Res Commun. 2020;526(1):98–104. doi:10.1016/j.bbrc.2020.03.054
47. Zhao P, Tang Z-W, Lin H-C, et al. VEGF aptamer/i-motif-based drug co-delivery system for combined chemotherapy and photodynamic therapy. Photodiagnosis Photodyn Ther. 2021;36:102547. doi:10.1016/j.pdpdt.2021.102547
48. Alzeibak R, Mishchenko TA, Shilyagina NY, et al. Targeting immunogenic cancer cell death by photodynamic therapy: past, present and future. J Immunother Cancer. 2021;9(1):e001926. doi:10.1136/jitc-2020-001926
49. Abrahamse H, Hamblin MR. New photosensitizers for photodynamic therapy. Biochem J. 2016;473(4):347–364. doi:10.1042/BJ20150942
50. Cozzani I, Jori G, Bertoloni G, et al. Efficient photosensitization of malignant human cells in vitro by liposome-bound porphyrins. Chem Biol Interact. 1985;53(1–2):131–143. doi:10.1016/s0009-2797(85)80091-0
51. Cao FQ, Yan MM, Liu YJ, et al. Photosensitizer-induced self-assembly of antigens as nanovaccines for cancer immunotherapy. Biomater Sci. 2018;6(3):473–477. doi:10.1039/c7bm01082f
52. Porcu EP, Salis A, Gavini E, et al. Indocyanine green delivery systems for tumour detection and treatments. Biotechnol Adv. 2016;34(5):768–789. doi:10.1016/j.biotechadv.2016.04.001
53. Liu Y, Dai S, Wen L, et al. Enhancing drug delivery for overcoming angiogenesis and improving the phototherapy efficacy of glioblastoma by ICG-loaded glycolipid-like micelles. Int J Nanomedicine. 2020;15:2717–2732. doi:10.2147/IJN.S234240
54. Yuan B, Wu H, Wang H, et al. A self-degradable supramolecular photosensitizer with high photodynamic therapeutic efficiency and improved safety. Angew Chem Int Ed Engl. 2021;60(2):706–710. doi:10.1002/anie.202012477
55. Li X, Zheng BY, Ke MR, et al. A tumor-pH-responsive supramolecular photosensitizer for activatable photodynamic therapy with minimal in vivo skin phototoxicity. Theranostics. 2017;7(10):2746–2756. doi:10.7150/thno.18861
56. Sun Q, He F, Sun C, et al. Honeycomb-satellite structured pH/H(2)O(2)-responsive degradable nanoplatform for efficient photodynamic therapy and multimodal imaging. ACS Appl Mater Interfaces. 2018;10(40):33901–33912. doi:10.1021/acsami.8b10207
57. Yang G, Xu L, Chao Y, et al. Hollow MnO(2) as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat Commun. 2017;8(1):902. doi:10.1038/s41467-017-01050-0
58. Feng C, Chen L, Lu Y, et al. Programmable Ce6 Delivery via Cyclopamine Based Tumor Microenvironment Modulating Nano-System for Enhanced Photodynamic Therapy in Breast Cancer. Front Chem. 2019;7:853. doi:10.3389/fchem.2019.00853
59. Kurian AW, Fish K, Shema SJ, Clarke CA. Lifetime risks of specific breast cancer subtypes among women in four racial/ethnic groups. Breast Cancer Res. 2010;12(6):R99. doi:10.1186/bcr2780
60. Churilla TM, Donnelly PE, Leatherman ER, et al. Total Mastectomy or Breast Conservation Therapy? How Radiation Oncologist Accessibility Determines Treatment Choice and Quality: a SEER Data-base Analysis. Breast J. 2015;21(5):473–480. doi:10.1111/tbj.12449
61. Liu W, Zhang K, Zhuang L, et al. Aptamer/photosensitizer hybridized mesoporous MnO(2) based tumor cell activated ROS regulator for precise photodynamic therapy of breast cancer. Colloids Surf B Biointerfaces. 2019;184:110536. doi:10.1016/j.colsurfb.2019.110536
62. Xu W, Qian J, Hou G, et al. A dual-targeted hyaluronic acid-gold nanorod platform with triple-stimuli responsiveness for photodynamic/photothermal therapy of breast cancer. Acta Biomater. 2019;83:400–413. doi:10.1016/j.actbio.2018.11.026
63. Dos Santos AF, De Almeida DRQ, Terra LF, et al. Fluence Rate Determines PDT Efficiency in Breast Cancer Cells Displaying Different GSH Levels. Photochem Photobiol. 2020;96(3):658–667. doi:10.1111/php.13182
64. Huang L, Lin H, Chen Q, et al. MPPa-PDT suppresses breast tumor migration/invasion by inhibiting Akt-NF-κB-dependent MMP-9 expression via ROS. BMC Cancer. 2019;19(1):1159. doi:10.1186/s12885-019-6374-x
65. Zou H, Wang F, Zhou JJ, et al. Application of photodynamic therapy for liver malignancies. J Gastrointest Oncol. 2020;11(2):431–442. doi:10.21037/jgo.2020.02.10
66. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. doi:10.3322/caac.21262
67. Shi R, Li C, Jiang Z, et al. Preclinical Study of Antineoplastic Sinoporphyrin Sodium-PDT via In Vitro and In Vivo Models. Molecules. 2017;22(1):112. doi:10.3390/molecules22010112
68. Barathan M, Mariappan V, Shankar E, et al. Hypericin-photodynamic therapy leads to interleukin-6 secretion by HepG2 cells and their apoptosis via recruitment of BH3 interacting-domain death agonist and caspases. Cell Death Dis. 2013;4(6):e697–e697. doi:10.1038/cddis.2013.219
69. Zhang H, Ma W, Li Y. Generation of effective vaccines against liver cancer by using photodynamic therapy. Lasers Med Sci. 2009;24(4):549–552. doi:10.1007/s10103-008-0609-4
70. Beik J, Abed Z, Ghoreishi FS, et al. Nanotechnology in hyperthermia cancer therapy: from fundamental principles to advanced applications. J Control Release. 2016;235:205–221. doi:10.1016/j.jconrel.2016.05.062
71. Bardhan R, Lal S, Joshi A, Halas NJ. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44(10):936–946. doi:10.1021/ar200023x
72. Zhao T, Qin S, Peng L, et al. Novel hyaluronic acid-modified temperature-sensitive nanoparticles for synergistic chemo-photothermal therapy. Carbohydr Polym. 2019;214:221–233. doi:10.1016/j.carbpol.2019.03.043
73. Wu S, Butt HJ. Near-Infrared-Sensitive Materials Based on Upconverting Nanoparticles. Adv Mater. 2016;28(6):1208–1226. doi:10.1002/adma.201502843
74. Sheng W, He S, Seare WJ, Almutairi A. Review of the progress toward achieving heat confinement-the holy grail of photothermal therapy. J Biomed Opt. 2017;22(8):80901. doi:10.1117/1.JBO.22.8.080901
75. Alkilany AM, Thompson LB, Boulos SP, et al. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64(2):190–199. doi:10.1016/j.addr.2011.03.005
76. Li J, Yu X, Jiang Y, et al. Second Near-Infrared Photothermal Semiconducting Polymer Nanoadjuvant for Enhanced Cancer Immunotherapy. Adv Mater. 2021;33(4):e2003458. doi:10.1002/adma.202003458
77. Kunjachan S, Detappe A, Kumar R, et al. Nanoparticle Mediated Tumor Vascular Disruption: a Novel Strategy in Radiation Therapy. Nano Lett. 2015;15(11):7488–7496. doi:10.1021/acs.nanolett.5b03073
78. Ali MR, Ali HR, Rankin CR, El-Sayed MA. Targeting heat shock protein 70 using gold nanorods enhances cancer cell apoptosis in low dose plasmonic photothermal therapy. Biomaterials. 2016;102:1–8. doi:10.1016/j.biomaterials.2016.06.017
79. Guo Z, Liu Y, Cheng X, et al. Versatile biomimetic cantharidin-tellurium nanoparticles enhance photothermal therapy by inhibiting the heat shock response for combined tumor therapy. Acta Biomater. 2020;110:208–220. doi:10.1016/j.actbio.2020.03.028
80. Wang S, Xin J, Zhang L, et al. Cantharidin-encapsulated thermal-sensitive liposomes coated with gold nanoparticles for enhanced photothermal therapy on A431 cells. Int J Nanomedicine. 2018;13:2143–2160. doi:10.2147/IJN.S156240
81. Wang S, Tian Y, Tian W, et al. Selectively Sensitizing Malignant Cells to Photothermal Therapy Using a CD44-Targeting Heat Shock Protein 72 Depletion Nanosystem. ACS Nano. 2016;10(9):8578–8590. doi:10.1021/acsnano.6b03874
82. Tang Y, Yang T, Wang Q, et al. Albumin-coordinated assembly of clearable platinum nanodots for photo-induced cancer theranostics. Biomaterials. 2018;154:248–260. doi:10.1016/j.biomaterials.2017.10.030
83. Liu T, Liu Z. 2D MoS(2) Nanostructures for Biomedical Applications. Adv Healthc Mater. 2018;7(8):e1701158. doi:10.1002/adhm.201701158
84. Tan C, Cao X, Wu XJ, et al. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem Rev. 2017;117(9):6225–6331. doi:10.1021/acs.chemrev.6b00558
85. Huang K, Li Z, Lin J, et al. Two-dimensional transition metal carbides and nitrides (MXenes) for biomedical applications. Chem Soc Rev. 2018;47(14):5109–5124. doi:10.1039/c7cs00838d
86. Gai S, Yang G. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today. 2018;19:146–187. doi:10.1016/j.nantod.2018.02.010
87. Chen H, Shao L, Li Q, Wang J. Gold nanorods and their plasmonic properties. Chem Soc Rev. 2013;42(7):2679–2724. doi:10.1039/c2cs35367a
88. He T, Jiang C, He J, et al. Manganese-Dioxide-Coating-Instructed Plasmonic Modulation of Gold Nanorods for Activatable Duplex-Imaging-Guided NIR-II Photothermal-Chemodynamic Therapy. Adv Mater. 2021;33(13):e2008540. doi:10.1002/adma.202008540
89. Kim D, Choi E, Lee C, et al. Highly sensitive and selective visual detection of Cr(VI) ions based on etching of silver-coated gold nanorods. Nano Converg. 2019;6(1):34. doi:10.1186/s40580-019-0206-1
90. Ye X, Zheng C, Chen J, et al. Using binary surfactant mixtures to simultaneously improve the dimensional tunability and monodispersity in the seeded growth of gold nanorods. Nano Lett. 2013;13(2):765–771. doi:10.1021/nl304478h
91. Yun Q, Xu J, Wei T, et al. Synthesis of Pd nanorod arrays on Au nanoframes for excellent ethanol electrooxidation. Nanoscale. 2022;14(3):736–743. doi:10.1039/d1nr05987d
92. Lee JH, Gibson KJ, Chen G, Weizmann Y. Bipyramid-templated synthesis of monodisperse anisotropic gold nanocrystals. Nat Commun. 2015;6:7571. doi:10.1038/ncomms8571
93. Liu Y, Wang Z, Liu Y, et al. Suppressing Nanoparticle-Mononuclear Phagocyte System Interactions of Two-Dimensional Gold Nanorings for Improved Tumor Accumulation and Photothermal Ablation of Tumors. ACS Nano. 2017;11(10):10539–10548. doi:10.1021/acsnano.7b05908
94. Song J, Yang X, Yang Z, et al. Rational Design of Branched Nanoporous Gold Nanoshells with Enhanced Physico-Optical Properties for Optical Imaging and Cancer Therapy. ACS Nano. 2017;11(6):6102–6113. doi:10.1021/acsnano.7b02048
95. Huang X, Tang S, Mu X, et al. Freestanding palladium nanosheets with plasmonic and catalytic properties. Nat Nanotechnol. 2011;6(1):28–32. doi:10.1038/nnano.2010.235
96. Tang S, Chen M, Zheng N. Sub-10-nm Pd nanosheets with renal clearance for efficient near-infrared photothermal cancer therapy. Small. 2014;10(15):3139–3144. doi:10.1002/smll.201303631
97. Dumas A, Couvreur P. Palladium: a future key player in the nanomedical field? Chem Sci. 2015;6(4):2153–2157. doi:10.1039/c5sc00070j
98. Chen YW, Su YL. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv Drug Deliv Rev. 2016;105(PtB):190–204. doi:10.1016/j.addr.2016.05.022
99. Yang K, Zhang S, Zhang G, et al. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 2010;10(9):3318–3323. doi:10.1021/nl100996u
100. Lin H, Wang Y, Gao S, et al. Theranostic 2D Tantalum Carbide (MXene). Adv Mater. 2020;32(42):e2003085. doi:10.1002/adma.202003085
101. Chen W, Ouyang J, Yi X, et al. Black Phosphorus Nanosheets as a Neuroprotective Nanomedicine for Neurodegenerative Disorder Therapy. Adv Mater. 2018;30:3. doi:10.1002/adma.201703458
102. Yang T, Tang Y, Liu L, et al. Size-Dependent Ag(2)S Nanodots for Second Near-Infrared Fluorescence/Photoacoustics Imaging and Simultaneous Photothermal Therapy. ACS Nano. 2017;11(2):1848–1857. doi:10.1021/acsnano.6b07866
103. Yang W, Guo W, Le W, et al. Albumin-Bioinspired Gd: cuSNanotheranostic Agent for In Vivo Photoacoustic/Magnetic Resonance Imaging-Guided Tumor-Targeted Photothermal Therapy. ACS Nano. 2016;10(11):10245–10257. doi:10.1021/acsnano.6b05760
104. Saeed M, Iqbal MZ, Ren W, et al. Controllable synthesis of Fe(3)O(4) nanoflowers: enhanced imaging guided cancer therapy and comparison of photothermal efficiency with black-TiO(2). J Mater Chem B. 2018;6(22):3800–3810. doi:10.1039/c8tb00745d
105. Li J, Rao J, Pu K. Recent progress on semiconducting polymer nanoparticles for molecular imaging and cancer phototherapy. Biomaterials. 2018;155:217–235. doi:10.1016/j.biomaterials.2017.11.025
106. Lei Z, Zhang F. Molecular Engineering of NIR-II Fluorophores for Improved Biomedical Detection. Angew Chem Int Ed Engl. 2021;60(30):16294–16308. doi:10.1002/anie.202007040
107. Yuan L, Lin W, Zheng K, et al. Far-red to near infrared analyte-responsive fluorescent probes based on organic fluorophore platforms for fluorescence imaging. Chem Soc Rev. 2013;42(2):622–661. doi:10.1039/c2cs35313j
108. Song X, Chen Q, Liu Z. Recent advances in the development of organic photothermal nano-agents. Nano Res. 2015;8(2):340–354. doi:10.1007/s12274-014-0620-y
109. Sheng Z, Hu D, Xue M, et al. Indocyanine green nanoparticles for theranostic applications. Nano-Micro Letters. 2013;5(3):145–150. doi:10.1007/BF03353743
110. Cai WR, Zhang GY, Lu KK, et al. Enhanced Electrochemiluminescence of One-Dimensional Self-Assembled Porphyrin Hexagonal Nanoprisms. ACS Appl Mater Interfaces. 2017;9(24):20904–20912. doi:10.1021/acsami.7b05188
111. Yang M, Deng J, Guo D, et al. Mitochondria-targeting Pt/Mn porphyrins as efficient photosensitizers for magnetic resonance imaging and photodynamic therapy. Dyes Pigments. 2019;166:189–195. doi:10.1016/j.dyepig.2019.03.048
112. Wu F, Yue L, Su H, et al. Carbon Dots @ Platinum Porphyrin Composite as Theranostic Nanoagent for Efficient Photodynamic Cancer Therapy. Nanoscale Res Lett. 2018;13(1):357. doi:10.1186/s11671-018-2761-5
113. Zhu S, Yao S, Wu F, et al. Platinated porphyrin as a new organelle and nucleus dual-targeted photosensitizer for photodynamic therapy. Org Biomol Chem. 2017;15(27):5764–5771. doi:10.1039/c7ob01003f
114. Xi D, Xiao M, Cao J, et al. NIR Light-Driving Barrier-Free Group Rotation in Nanoparticles with an 88.3% Photothermal Conversion Efficiency for Photothermal Therapy. Adv Mater. 2020;32(11):e1907855. doi:10.1002/adma.201907855
115. He K, Chen S, Xu W, et al. High-stability NIR-II fluorescence polymer synthesized by atom transfer radical polymerization for application in high-resolution NIR-II imaging. Biomater Sci. 2021;9(19):6434–6443. doi:10.1039/d1bm01074c
116. Nagarajan K, Mallia AR, Muraleedharan K, Hariharan M. Enhanced intersystem crossing in core-twisted aromatics. Chem Sci. 2017;8(3):1776–1782. doi:10.1039/c6sc05126j
117. Li C, Luo Z, Yang L, et al. Self-assembled porphyrin polymer nanoparticles with NIR-II emission and highly efficient photothermal performance in cancer therapy. Mater Today Bio. 2022;13:100198. doi:10.1016/j.mtbio.2021.100198
118. Jung HS, Verwilst P, Sharma A, et al. Organic molecule-based photothermal agents: an expanding photothermal therapy universe. Chem Soc Rev. 2018;47(7):2280–2297. doi:10.1039/c7cs00522a
119. Gao D, Zhang B, Liu Y, et al. Molecular Engineering of Near-Infrared Light-Responsive BODIPY-Based Nanoparticles with Enhanced Photothermal and Photoacoustic Efficiencies for Cancer Theranostics. Theranostics. 2019;9(18):5315–5331. doi:10.7150/thno.34418
120. Liu N, Oconnor P, Gujrati V, et al. Facile synthesis of a croconaine-based nanoformulation for optoacoustic imaging and photothermal therapy. Adv Healthc Mater. 2021;10(9):e2002115. doi:10.1002/adhm.202002115
121. Zhang Q, Guo Q, Chen Q, et al. Highly Efficient 2D NIR-II Photothermal Agent with Fenton Catalytic Activity for Cancer Synergistic Photothermal-Chemodynamic Therapy. Adv Sci. 2020;7(7):1902576. doi:10.1002/advs.201902576
122. Qiu T, Lan Y, Wei Z, et al. In vivo Multi-scale Photoacoustic Imaging Guided Photothermal Therapy of Cervical Cancer based on Customized Laser System and Targeted Nanoparticles. Int J Nanomedicine. 2021;16:2879–2896. doi:10.2147/IJN.S301664
123. Yang H, Cao Y, Li D, et al. AS1411 and EpDT3-conjugated silver nanotriangle-mediated photothermal therapy for breast cancer and cancer stem cells. Nanomedicine. 2021;16(28):2503–2519. doi:10.2217/nnm-2021-0257
124. Zhou Z, Yan Y, Hu K, et al. Autophagy inhibition enabled efficient photothermal therapy at a mild temperature. Biomaterials. 2017;141:116–124. doi:10.1016/j.biomaterials.2017.06.030
125. Kang X, Sun T, Zhang L, et al. Synergistic Theranostics of Magnetic Resonance Imaging and Photothermal Therapy of Breast Cancer Based on the Janus Nanostructures Fe(3)O(4)-Au(shell)-PEG. Int J Nanomedicine. 2021;16:6383–6394. doi:10.2147/IJN.S322894
126. Liu T, Song Y, Huang Z, et al. Photothermal photodynamic therapy and enhanced radiotherapy of targeting copolymer-coated liquid metal nanoparticles on liver cancer. Colloids Surf B Biointerfaces. 2021;207:112023. doi:10.1016/j.colsurfb.2021.112023
127. Zhu H, Li Y, Ming Z, Liu W. Glucose oxidase-mediated tumor starvation therapy combined with photothermal therapy for colon cancer. Biomater Sci. 2021;9(16):5577–5587. doi:10.1039/d1bm00869b
128. Gadeval A, Chaudhari S, Bollampally SP, et al. Integrated nanomaterials for non-invasive photothermal therapy of rheumatoid arthritis. Drug Discov Today. 2021;26(10):2315–2328. doi:10.1016/j.drudis.2021.04.026
129. Farhood B, Najafi M, Mortezaee K. CD8(+) cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol. 2019;234(6):8509–8521. doi:10.1002/jcp.27782
130. Tan S, Li D, Zhu X. Cancer immunotherapy: pros, cons and beyond. Biomed Pharmacother. 2020;124:109821. doi:10.1016/j.biopha.2020.109821
131. Chen Q, Xu L, Liang C, et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat Commun. 2016;7:13193. doi:10.1038/ncomms13193
132. Li B, Chan HL, Chen P. Immune Checkpoint Inhibitors: basics and Challenges. Curr Med Chem. 2019;26(17):3009–3025. doi:10.2174/0929867324666170804143706
133. Li X, Lovell JF, Yoon J, Chen X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat Rev Clin Oncol. 2020;17(11):657–674. doi:10.1038/s41571-020-0410-2
134. Fan W, Yung B, Huang P, Chen X. Nanotechnology for Multimodal Synergistic Cancer Therapy. Chem Rev. 2017;117(22):13566–13638. doi:10.1021/acs.chemrev.7b00258
135. Tan K, Li R, Huang X, Liu Q. Outer membrane vesicles: current status and future direction of these novel vaccine adjuvants. Front Microbiol. 2018;9:783. doi:10.3389/fmicb.2018.00783
136. Kumar V. Corrigendum to ‘Toll-like receptors in immunity and inflammatory diseases: past, present, and future. Int Immunopharmacol. 2018;62:338. doi:10.1016/j.intimp.2018.06.044
137. Bahmani B, Gong H, Luk BT, et al. Intratumoral immunotherapy using platelet-cloaked nanoparticles enhances antitumor immunity in solid tumors. Nat Commun. 2021;12(1):1999. doi:10.1038/s41467-021-22311-z
138. Xu J, Xu L, Wang C, et al. Near-Infrared-Triggered Photodynamic Therapy with Multitasking Upconversion Nanoparticles in Combination with Checkpoint Blockade for Immunotherapy of Colorectal Cancer. ACS Nano. 2017;11(5):4463–4474. doi:10.1021/acsnano.7b00715
139. Hwang HS, Cherukula K, Bang YJ, et al. Combination of Photodynamic Therapy and a Flagellin-Adjuvanted Cancer Vaccine Potentiated the Anti-PD-1-Mediated Melanoma Suppression. Cells. 2020;9:11. doi:10.3390/cells9112432
140. Jo Y, Ali LA, Shim JA, et al. Innovative CAR-T Cell Therapy for Solid Tumor; Current Duel between CAR-T Spear and Tumor Shield. Cancers. 2020;12(8):2087. doi:10.3390/cancers12082087
141. Korbelik M, Banáth J, Zhang W, et al. N-dihydrogalactochitosan as immune and direct antitumor agent amplifying the effects of photodynamic therapy and photodynamic therapy-generated vaccines. Int Immunopharmacol. 2019;75:105764. doi:10.1016/j.intimp.2019.105764
142. Mo Z, Yu F, Han S, et al. New peptide MY1340 revert the inhibition effect of VEGF on dendritic cells differentiation and maturation via blocking VEGF-NRP-1 axis and inhibit tumor growth in vivo. Int Immunopharmacol. 2018;60:132–140. doi:10.1016/j.intimp.2018.04.025
143. Huang Y, Kim BYS, Chan CK, et al. Improving immune-vascular crosstalk for cancer immunotherapy. Nat Rev Immunol. 2018;18(3):195–203. doi:10.1038/nri.2017.145
144. Cramer GM, Moon EK, Cengel KA, Busch TM. Photodynamic Therapy and Immune Checkpoint Blockade. Photochem Photobiol. 2020;96(5):954–961. doi:10.1111/php.13300
145. Kim S, Kim SA, Nam GH, et al. In situ immunogenic clearance induced by a combination of photodynamic therapy and rho-kinase inhibition sensitizes immune checkpoint blockade response to elicit systemic antitumor immunity against intraocular melanoma and its metastasis. J Immunother Cancer. 2021;9(1):e001481. doi:10.1136/jitc-2020-001481
146. Yuan Z, Fan G, Wu H, et al. Photodynamic therapy synergizes with PD-L1 checkpoint blockade for immunotherapy of CRC by multifunctional nanoparticles. Mol Ther. 2021;29(10):2931–2948. doi:10.1016/j.ymthe.2021.05.017
147. Liu X, Liu Y, Li X, et al. ER-Targeting PDT Converts Tumors into In Situ Therapeutic Tumor Vaccines. ACS Nano. 2022. doi:10.1021/acsnano.2c01669
148. Zhang H, Wang P, Wang X, et al. Antitumor Effects of DC Vaccine With ALA-PDT-Induced Immunogenic Apoptotic Cells for Skin Squamous Cell Carcinoma in Mice. Technol Cancer Res Treat. 2018;17:1533033818785275. doi:10.1177/1533033818785275
149. Trempolec N, Doix B, Degavre C, et al. Photodynamic Therapy-Based Dendritic Cell Vaccination Suited to Treat Peritoneal Mesothelioma. Cancers. 2020;12(3):545. doi:10.3390/cancers12030545
150. Gunaydin G, Gedik ME, Ayan S. Photodynamic therapy for the treatment and diagnosis of cancer-a review of the current clinical status. Front Chem. 2021;9:686303. doi:10.3389/fchem.2021.686303
151. Doix B, Trempolec N, Riant O, Feron O. Low Photosensitizer Dose and Early Radiotherapy Enhance Antitumor Immune Response of Photodynamic Therapy-Based Dendritic Cell Vaccination. Front Oncol. 2019;9:811. doi:10.3389/fonc.2019.00811
152. Chu KF, Dupuy DE. Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer. 2014;14(3):199–208. doi:10.1038/nrc3672
153. Shi H, Cao T, Connolly JE, et al. Hyperthermia enhances CTL cross-priming. J Immunol. 2006;176(4):2134–2141. doi:10.4049/jimmunol.176.4.2134
154. Sun W, Du Y, Liang X, et al. Synergistic triple-combination therapy with hyaluronic acid-shelled PPy/CPT nanoparticles results in tumor regression and prevents tumor recurrence and metastasis in 4T1 breast cancer. Biomaterials. 2019;217:119264. doi:10.1016/j.biomaterials.2019.119264
155. Chen Q, Chen J, Yang Z, et al. Nanoparticle-Enhanced Radiotherapy to Trigger Robust Cancer Immunotherapy. Adv Mater. 2019;31(10):e1802228. doi:10.1002/adma.201802228
156. Zhou T, Liang X, Wang P, et al. A Hepatocellular Carcinoma Targeting Nanostrategy with Hypoxia-Ameliorating and Photothermal Abilities that, Combined with Immunotherapy, Inhibits Metastasis and Recurrence. ACS Nano. 2020;14(10):12679–12696. doi:10.1021/acsnano.0c01453
157. Lima Sousa R, Melo BL, Alves CG, et al. Combining Photothermal‐Photodynamic Therapy Mediated by Nanomaterials with Immune Checkpoint Blockade for Metastatic Cancer Treatment and Creation of Immune Memory. Adv Funct Mater. 2021;31(29):2010777. doi:10.1002/adfm.202010777
158. Yan S, Zeng X, Tang Y, et al. Activating Antitumor Immunity and Antimetastatic Effect Through Polydopamine-Encapsulated Core-Shell Upconversion Nanoparticles. Adv Mater. 2019;31(46):e1905825. doi:10.1002/adma.201905825
159. Yu J, He X, Wang Z, et al. Combining PD-L1 inhibitors with immunogenic cell death triggered by chemo-photothermal therapy via a thermosensitive liposome system to stimulate tumor-specific immunological response. Nanoscale. 2021;13(30):12966–12978. doi:10.1039/d1nr03288g
160. Huang J, Zhang L, Zhou W, et al. Dual mitigation of immunosuppression combined with photothermal inhibition for highly effective primary tumor and metastases therapy. Biomaterials. 2021;274:120856. doi:10.1016/j.biomaterials.2021.120856
161. Nam J, Son S, Ochyl LJ, et al. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat Commun. 2018;9(1):1074. doi:10.1038/s41467-018-03473-9
162. Nam J, Son S, Park KS, Moon JJ. Photothermal therapy combined with neoantigen cancer vaccination for effective immunotherapy against large established tumors and distant metastasis. Adv Ther. 2021;4:8. doi:10.1002/adtp.202100093
163. Qiu J, Xiao Q, Zheng X, et al. Single W18O49 nanowires: a multifunctional nanoplatform for computed tomography imaging and photothermal/photodynamic/radiation synergistic cancer therapy. Nano Res. 2015;8(11):3580–3590. doi:10.1007/s12274-015-0858-z
164. Wang H, Pan X, Wang X, et al. Degradable Carbon-Silica Nanocomposite with Immunoadjuvant Property for Dual-Modality Photothermal/Photodynamic Therapy. ACS Nano. 2020;14(3):2847–2859. doi:10.1021/acsnano.9b06168
165. Liang R, Liu L, He H, et al. Oxygen-boosted immunogenic photodynamic therapy with gold [email protected] dioxide to inhibit tumor growth and metastases. Biomaterials. 2018;177:149–160. doi:10.1016/j.biomaterials.2018.05.051
166. Hameed S, Mo S, Mustafa G, et al. Immunological Consequences of Nanoparticle‐Mediated Antitumor Photoimmunotherapy. Adv Therapeutics. 2020;3(5):1900101. doi:10.1002/adtp.201900101
167. Zhang X, Tang J, Li C, et al. A targeting black phosphorus nanoparticle based immune cells nano-regulator for photodynamic/photothermal and photo-immunotherapy. Bioact Mater. 2021;6(2):472–489. doi:10.1016/j.bioactmat.2020.08.024
168. Hu C, Cai L, Liu S, et al. Copper-doped nanoscale covalent organic polymer for augmented photo/chemodynamic synergistic therapy and immunotherapy. Bioconjug Chem. 2020;31(6):1661–1670. doi:10.1021/acs.bioconjchem.0c00209
169. Naylor MF, Chen WR, Teague TK, Perry LA, Nordquist RE. In situ photoimmunotherapy: a tumour-directed treatment for melanoma. Br J Dermatol. 2006;155(6):1287–1292. doi:10.1111/j.1365-2133.2006.07514.x
170. Mroz P, Hashmi JT, Huang YY, Lange N, Hamblin MR. Stimulation of anti-tumor immunity by photodynamic therapy. Expert Rev Clin Immunol. 2011;7(1):75–91. doi:10.1586/eci.10.81
171. Santos LL, Oliveira J, Monteiro E, Santos J, Sarmento C. Treatment of head and neck cancer with photodynamic therapy with redaporfin: a clinical case report. Case Rep Oncol. 2018;11(3):769–776. doi:10.1159/000493423
172. Jiang R, Dai J, Dong X, et al. Improving Image-Guided Surgical and Immunological Tumor Treatment Efficacy by Photothermal and Photodynamic Therapies Based on a Multifunctional NIR AIEgen. Adv Mater. 2021;33(22):e2101158. doi:10.1002/adma.202101158
173. Wang Y, Zhang L, Zhao G, et al. Homologous targeting nanoparticles for enhanced PDT against osteosarcoma HOS cells and the related molecular mechanisms. J Nanobiotechnology. 2022;20(1):83. doi:10.1186/s12951-021-01201-y
174. Huang X, Chen L, Lin Y, et al. Tumor targeting and penetrating biomimetic mesoporous polydopamine nanoparticles facilitate photothermal killing and autophagy blocking for synergistic tumor ablation. Acta Biomater. 2021;136:456–472. doi:10.1016/j.actbio.2021.09.030
175. Wang X, Liu Y, Xue C, et al. A protein-based cGAS-STING nanoagonist enhances T cell-mediated anti-tumor immune responses. Nat Commun. 2022;13(1):5685. doi:10.1038/s41467-022-33301-0
176. Zhou B, Sun X, Dong B, et al. Antibacterial PDT nanoplatform capable of releasing therapeutic gas for synergistic and enhanced treatment against deep infections. Theranostics. 2022;12(6):2580–2597. doi:10.7150/thno.70277
177. Zhang Y, Feng Y, Huang Y, et al. Tumor-Targeted Gene Silencing IDO Synergizes PTT-Induced Apoptosis and Enhances Anti-tumor Immunity. Front Immunol. 2020;11:968. doi:10.3389/fimmu.2020.00968
178. Li W, Yang J, Luo L, et al. Targeting photodynamic and photothermal therapy to the endoplasmic reticulum enhances immunogenic cancer cell death. Nat Commun. 2019;10(1):3349. doi:10.1038/s41467-019-11269-8



