Ferroptosis: Reviewing CRC with the Third Eye
Introduction
Colorectal cancer (CRC) is one of the most common cancers and it has become the second leading cause of cancer death which incidence increasing each year.1 In addition to genetic risk and environmental factors, unhealthy diet and lifestyle factors may modify the risk of CRC.2–4 Although early screening and diagnosis have improved survival, side effects accompanying conventional treatment seriously affect the quality of life. Tumor recurrence and metastasis as well as drug resistance have become major challenges for CRC therapy, so new treatments are urgently needed to improve the survival and prognosis of CRC patients. Given that CRC is the only type of malignancy with two iron uptake pathways, from both the intestinal lumen and blood, we infer that focus on ferroptosis may become a breakthrough point for CRC treatments. So far, a series of studies have corroborated the relationship between CRC cells and ferroptosis.
Ferroptosis is a form of regulated cell death (RCD) that differs from other types of cell death in terms of morphology, biochemistry, and genetics. Ferroptosis was first proposed by Dixon and his colleagues in 2012.5 In 2018, the Nomenclature Committee on Cell Death (NCCD) added ferroptosis to the RCD family.6 Morphologically, ferroptosis is characterized by smaller mitochondria with condensed mitochondrial membrane densities, reduced or vanished mitochondrial cristae, and rupture of the outer mitochondrial membrane.
Ferroptosis is an emerging field that has quickly updated development, with the in-depth study of its mechanisms and regulation, there have been connections verified between ferroptosis and pathophysiologic phenomena. Over the past few years, we have found the trail of ferroptosis in a variety of malignancies, in which ferroptosis is involved in their progression and influenced therapeutic response.7 More and more drugs, chemicals, and bioactive products in the body or extracted from plants have shown a regulatory effect on ferroptosis. In this review, we summarize the progression of mechanisms and regulatory pathways of ferroptosis in CRC and elaborate on their associations under the corroboration of bioinformatics analysis and diverse experimental data. We also emphasize the potential of ferroptosis working as a novel strategy for therapy in CRC.
Mechanisms of Ferroptosis
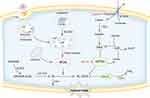
Ferroptosis was originally defined as iron-dependent lipid peroxidation. For ferroptosis to occur, specific lipids must undergo oxidation. That is to say, the natural defenses that block the accumulation of oxidized lipids and repair the damaged membrane must become compromised. Ultimately, accumulated lipid peroxide stands out in the competition between the oxidative system and the reduction system, leading to plasma membrane damage and cell death. Figure 1 Iron and lipid peroxide released from ferroptotic cells also act as signals to wave-like propagation of ferroptosis in the cell population.8
Oxidative Damage
Iron Accumulation and ROS Production
Iron homeostasis is critical for ferroptosis regulation. Iron is an essential element for cellular homeostasis and organismal survival, and it is involved in a series of crucial physiological processes such as oxygen transport, oxidative phosphorylation, and enzymatic function. Ferritin is the major form of iron storage, which contains two components, including ferritin light chain (FTL) and ferritin heavy chain 1 (FTH1). Most of the iron in the systemic circulation is present as trivalent iron (Fe3+) bound to transferrin (TF). Then, on the cell membrane, the transferrin receptor (TFRC) recognizes iron-containing TF and transports it into the cell via endosomes, where STEP3 as a ferrireductase reduces Fe3+ to ferrous iron (Fe2+). Then, with the assistance of SLC11A2 (also known as DMT1), Fe2+ is released from the endosome, and maintained in the form of labile iron pool (LIP) or transferred to the extracellular milieu through the iron-efflux protein solute carrier family 40 member 1(SLC40A1/ferroportin1).9 Furthermore, the ferritin complex is transported to autophagosomes and undergoes degradation by nuclear receptor coactivator 4 (NCOA4), thus increasing cellular labile iron levels.10 Then, through the Fenton reaction, high levels of intracellular Fe2+ generate excess reactive oxygen species (ROS) which finally induce lipid peroxidation.11 And naturally, iron chelators that bind free iron can protect against ferroptosis by reducing levels of intracellular Fe2+.
ROS is a ubiquitous oxidation product of cellular metabolism that is primarily generated during oxidative phosphorylation in the mitochondria. It plays a pivotal role in stress response and acts as an important second messenger in signaling transduction. Under normal circumstances, intracellular ROS levels are maintained in a low physiological range. Mild elevation of ROS can promote tumor cell proliferation, invasion, and migration, and may also contribute to apoptosis evasion, thus leading to drug resistance.12 While excess ROS initiates the production of lipid peroxides (LOOH) in the membrane phospholipids, leading to the damage of membrane structure and function. Above all, iron accumulation and ROS production are key steps to trigger ferroptosis.
Moreover, cancer cells, especially cancer stem cells, need a large amount of iron to sustain their rapid proliferation.13 They also have high basal levels of ROS as a result of their exuberant cell metabolism and proliferation. CRC is the only type of malignancy with two iron uptake resources, whose iron comes from the intestinal cavity and systemic circulation. And there have been reports suggesting that high dietary iron elevates the risk of CRC for humans.14 A recent study also shows that iron exposure could activate ROS and enhance the Warburg effect to protect CRC from ferroptosis.15 This evidence revealed a close link between ferroptosis and CRC and shed light on new therapeutic ideas.
Lipid Peroxidation
The accumulation of lipid peroxidation products is the central process of ferroptosis by which oxidants attack the carbon–carbon double bonds of lipids. The primary substrates of lipid peroxidation are polyunsaturated fatty acids (PUFAs).16 Several biological processes, for example, cellular growth, immunity regulation, and inflammation are regulated by PUFAs, which are components of the cell membrane. The unstable double bonds in the structure of PUFA make it sensitive to oxidation and their number largely determined the susceptibility. To drive ferroptosis, PUFAs need to be activated and inserted into membrane lipids.17 The two primary PUFAs that trigger ferroptosis are arachidonic acid (AA) and adrenal acid (AdA). Either enzyme catalysis or non-enzymatic-free radical-chain reaction can mediate PUFA oxidation. Free PUFAs are attached to CoA by Acyl-CoA Synthetase Long-Chain Family Member 4 (ACSL4), and then incorporated into membrane phospholipids by Lysophosphatidylcholine Acyltransferase 3 (LPCAT3).18 Some certain lipoxygenases (LOXs) can directly catalyze lipid peroxidation in the membrane.19 Lysophospholipids can be oxidized by prostaglandin-endoperoxide synthase 2/cyclooxygenase-2 (PTGS2/COX2), which is regarded as a biomarker of ferroptosis.20 Extensive lipid peroxidation can destroy membrane structure and increase its permeability. It also causes the generation of numerous toxic byproducts including 4-hydroxynonenal (4-HNE) and malondialdehyde (MDA). They can produce extremely toxic cytotoxicity when they interact with proteins, DNA bases, and other nucleophilic molecules.21
Antioxidants and Oxidative Damage-Repairing Systems
GPX4 and GSH-Related Axis
An antioxidant enzyme, called glutathione peroxidase 4 (GPX4), mediates enzymatic antioxidant processes, which is a crucial defense mechanism against lipid peroxidation. By binding to reduced glutathione (GSH), it transforms toxic lipid peroxidation into harmless lipid alcohols, while producing oxidized glutathione (GSSG) to resist lipid peroxidation. GSH is a synthetic substrate and cofactor for GPX4. The cytosolic enzymes glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS) catalyze the tripeptide’s synthesis from glutamate, cysteine, and glycine. Glutamate-cysteine ligase catalytic subunit (GCLC) and glutamate-cysteine ligase modifying subunit (GCLM) make up GCL, which operates as a rate-limiting enzyme in the production of GSH. Cysteine is also considered a rate-limiting precursor of GSH synthesis due to its limited concentration. Cysteine is imported into the cell in its oxidized form via system xc-, which consists of two subunits including SLC7A11 and SLC3A2. The former mediates the antiporter activity and the latter maintains its protein stability and appropriate localization. And then, cystine is immediately reduced to cysteine. Erastin blocks SLC7A11 and causes GSH depletion, while RSL3 inactivates GPX4. Both of them are commonly used ferroptosis inducers in relevant experiments.20,22
It is reported that colorectal cancer stem cells (CSCs) have lower levels of ROS and higher levels of cysteine, GSH, and SLC7A11 compared to normal CRC cells, and knockdown of SLC7A11 notably raise the ROS level and reduce cysteine, GSH levels, and then attenuates the activity of CSCs. Erastin also has significantly stronger cytotoxic effects on CSCs that can be used to inhibit CRC progression and drug resistance.23 RSL3 also showed its emerging role in CRC ferroptosis through GPX4 inactivation and ROS production.24
GPX4-Independent Defense for Ferroptosis
Although GPX4 is the main defense against lipid peroxidation, ubiquinone (Coenzyme Q10, CoQ10) can directly scavenge lipid peroxyl radicals and act as a secondary endogenous defense against lipid peroxidation.25 And utilizing NAD(P)H, ferroptosis suppressor protein (FSP1) plays a role in catalyzing the regeneration of non-mitochondrial CoQ10.26 Additionally, GCH1 (GTP cyclo- hydrolase 1) may also produce the metabolic byproducts tetrahydrobiopterin/dihydrobiopterin (BH4/BH2) and regulate the formation of CoQ10 to prevent ferroptosis.27 Suppressing the GCH1/BH4 metabolism can activate NCOA4-mediated ferritinophagy to promote ferroptosis.28 Besides, ESCRT-III is also a commonly accepted mechanism to repair membrane damage after different kinds of cell death, for example, necrosis and pyroptosis. It is also reported to protect tumor cells after being attacked by T cells. The increasing cytosolic Ca2+ appeared after lipid peroxidation and was also a hallmark of plasma membrane damage and ferroptosis. And the ESCRT-III complex will be activated by the increasing Ca2+ to mediate membrane repair.29
Regulation of Ferroptosis in CRC
Gene
Genes encode and modulate the expression of proteins and are involved in various cellular activities. By integrating the clinical data of CRC patients and gene expression profiles from public databases, and verifying through gene technologies, a series of genes and signal pathways are proposed to engage in the regulation of ferroptosis in CRC and may be used to predict the prognosis of the disease. Inhibiting SRSF9 can downregulate GPX4 levels and increase ferroptosis induced by erastin in CRC cells.30 Liu et al performed both bioinformatics analysis and in vitro experiments to investigate the biological function and signal regulation pathway of Solute carrier family 2 member 1 (SLC2A1), which encodes a glucose transporter (GLUT) and is involved in multiple physiological and pathophysiological processes. They validated that SLC2A1 was strongly expressed in CRC and connected to a series of regulatory networks for example ferroptosis, which makes it a potential diagnostic biomarker.31 Peng et al explored a ferroptosis-related gene (FRG), Metallothionein-1G (MG1T), and demonstrated that CRC patients’ immune responses may be impacted by decreased levels of MG1T, which already showed a worse prognosis in CRC patients.32 There are also some other FRGs screened from databases that are closely related to ferroptosis in CRC, for example, CYBB, YAP1,33 NFS1,34 TFAP2C, ALOX12, NOS2,35–37 NOX437,38 and so on. While these results may lack universality and need to be further verified.
One of the most thoroughly investigated traditional tumor suppressor genes is tumor protein 53 (TP53). About 60% of CRC patients had P53 mutations. The mutant P53 activates oncogenic and inflammatory pathways, giving tumor cells the capacity to invade and spread, thus accelerating the progression of advanced CRC. P53 has a double-side ferroptosis regulation mode depending on different cancer types and intracellular metabolic states.39,40 P53 blocks the transcription of SLC7A11 to suppress cystine uptake and induce GSH depletion and ferroptosis in some types of human cancer cells.41 However, TP53 was found to suppress ferroptosis in CRC cells by binding to dipeptidyl-peptidase 4 (DPP4). DPP4 has peptidase activity and can mediate ROS production to regulate lipid metabolism and ferroptosis. Loss of TP53 increased DPP4 pathway activation, triggered lipid peroxidation, and made human CRC cells more susceptible to ferroptosis induced by erastin in vivo.42
Noncoding RNA
Long Noncoding RNA
Transcripts with a length of more than 200 nucleotides that do not code for proteins are known as long non-coding RNA (lncRNA).43 LncRNA regulates gene expression at epigenetic, post-transcriptional, and transcriptional levels.44 It is an important factor in tumors’ biological processes such as cell proliferation, differentiation, and migration.45 Increasing studies have confirmed that lncRNA could regulate MicroRNA (miRNA) by acting as competing endogenous RNA (ceRNA) in cancer progression.46 LNC01606 was frequently at a highly expressed level in CRC and closely linked to a poor prognosis. Mechanically, LNC01606 served as a ceRNA to modulate the expression of miR-423-5p, which would enhance the stearoyl-CoA desaturase 1 (SCD1) expression and activate the classical Wnt/β-catenin signaling, subsequently inhibiting ferroptosis and enhancing stemness.47
Although there are no more reported mechanisms of specific lncRNA regulating ferroptosis in CRC, lncRNA can serve as valuable prognostic markers. Researchers analyzed lncRNA expression data profiles and integrated clinical data, thereby constructing CRC prognostic risk models based on ferroptosis-related lncRNAs.
To assess the prognosis of patients suffering from colon adenocarcinoma (COAD), Zeng et al created a differentially expressed lncRNA signature and found that four lncRNAs (LINC01555, RP11-610P16.1, RP11-108K3.1, and LINC01207) were identified to possess the most remarkable correlation with survival in COAD.48 Zhang et al developed a nomogram including age, T stage, pathologic stage, and risk score that has great predictive power. They also developed a ferroptosis-related lncRNA prognosis signature in CRC that consists of VPS9D1-AS1, ELFN1-AS1, AC099850.3, and AC016027.1.49 These researches filtered out some ferroptosis-related lncRNAs that were highly related to CRC prognosis and revealed potential signal pathways among them. These models not only present individualized predictions for prognosis but also provide promising targets for exploring the mechanisms of ferroptosis and CRC.
MiRNA
MiRNAs are a series of tiny single-stranded RNA molecules of 18–25 nucleotides, which are highly conserved yet do not have an encoding function. They repress translation and regulate gene expression levels by binding the sequences in the 3’-untranslated region of messenger RNAs (mRNAs). And by binding with various mRNAs and blocking post-transcriptional expression of specific genes, miRNAs play different roles in regulating ferroptosis.
MiR-15a-3p could directly target GPX4 and result in sensitivity to ferroptosis in CRC cells.50 MiR-539 can stimulate the SAPK/JAK axis, which will promote ferroptosis in vitro and inhibit tumor growth in vivo.51 On the contrary, miR-545 targets the TF genes to suppress ferroptosis and play an oncogenic role in CRC cells. Nude mice with implanted miR-545 knocked down CRC cells were found with decreased tumor size after erastin treatment.52
Using whole-transcriptome profiling and anti-correlation analysis, Angius et al thoroughly analyzed the miRNA expression profiles of CRC and healthy colon tissue to determine the genes that each deregulated miRNA was targeting. Their research characterized numerous pathways involved in the development of CRC and discovered an integrated signature of 20 deregulated miRNAs in CRC.53
CircRNA
Circular RNAs (circRNAs) are covalently closed loops that are generated by the back splicing of pre-mRNAs. CircRNAs can function as sponges of miRNAs to regulate gene expression and then the pathogenesis of tumors.54 Their abundant presence, relatively high stability, and widespread expression make them potential diagnostic and prognostic biomarkers for cancers.55
Circ_0007142 was found as a sponge of miR-874-3p, which directly repressed glycerophosphodiester phosphodiesterase domain containing 5 (GDPD5) expression and acted as a ferroptosis promoter and a tumor inhibitor in CRC.56 Circular ATP binding cassette subfamily B member 10 (circABCB10) serves as a sponge of miR-326 to regulate C-C motif chemokine ligand 5 (CCL5) expression in CRC cells. CircABCB10 and CCL5 were highly expressed in CRC. CircABCB10 knockdown promoted ferroptosis in vitro and suppressed tumor growth in vivo, indicating its potential as a therapeutic target for CRC therapy.57
Nrf2
The transcription factor nuclear factor-erythroid 2-related factor 2 (Nrf2) is considered a pivotal mechanism in regulating redox balance. Normally, Nrf2 binds primarily to Kelch-like ECH-associated protein 1 (KEAP1), inhibits its activation, and undergoes constant ubiquitination proteasome and degradation, which maintains Nrf2 at a basally low level.58 When cells are under stress conditions induced by ROS or electrophilic substances, Nrf2 is activated after dissociating from KEAP1. Then, Nrf2 enters the nucleus and binds to the antioxidant response element (ARE) sequence, leading to the production of cellular defense-related proteins and enzymes.59 Nrf2 protects cells from ferroptosis by regulating relative genes. NRF2 positively regulates xCT, GCLC, and GCLM, three genes involved in the synthesis of GSH.60–62 NRF2 also promotes the expression of FTH1 and FTL.63 A vital NRF2-controlled enzyme is called heme oxygenase-1 (HO-1), which can catalyze the degradation of heme into iron, biliverdin, and carbon monoxide (CO). The first two products are strong antioxidants to protect against ferroptosis, while the latter promotes ferroptosis. HO-1 has been confirmed to be a double-edged sword in regulating ferroptosis.63,64
Cetuximab inhibits the Nrf2/HO-1 axis thus enhancing RSL3-induced ferroptosis.65 However, Tagitinin C, a sesquiterpene lactone, induces oxidative stress and activates the Nrf2/HO-1 pathway. The increased HO-1 expression leads to the increased LIP and promotes lipid peroxidation, ultimately inducing ferroptosis in CRC cells.66 Likewise, the B. etnensis Raf. extract promotes the oxidation degree of the cellular redox environment, stimulates ferroptosis, and causes CRC cell death by increasing HO-1 expression.67
Other Signaling Regulatory Pathways
The activation of transcription factors hypoxia-inducible factor 2α (HIF-2α) can upregulate cellular iron levels and enhance irreversible cysteine oxidation, thereby inducing ferroptosis in CRC cells. Knockdown or inhibition of HIF-2α leads to reduced ROS and tolerance to ferroptosis in vivo and in vitro, which can be used for CRC therapy.68
OTUD1 was found to be the deubiquitinase of iron-responsive element-binding protein 2 (IREB2). OTUD1 stimulates ROS generation and ferroptosis by deubiquitinating and stabilizing IREB2 and promoting iron transportation. Downregulation of OTUD1 limits the accumulation of tumor-reactive T cells, which also makes it correlated with poor outcomes of CRC.69
Additionally, ferroptosis has some links with other types of regulated cell death. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) and ferroptosis inducers like erastin and artesunate (ART) exerted synergic action in CRC cell death. Erastin and ART can stimulate the C/EBP-homologous protein (CHOP), leading to endoplasmic reticulum stress and the expression of p53 upregulated modulator of apoptosis (PUMA), which is a potent pro-apoptosis factor. The lack of CHOP and PUMA, but not p53 downstream eliminates the synergism of ferroptosis inducers, indicating that the CHOP/PUMA axis also affects the function of ferroptosis inducers.70 These findings remind us of a new angle to implore the crosswalk between ferroptosis and other forms of RCD.
Bright Future of CRC Therapy
Ferroptosis and Tumor Drug Resistance
Drug resistance and cancer metastasis are major obstacles in CRC treatment and profoundly influence the therapeutic efficiency and prognosis. A better understanding of the mechanisms and regulation of ferroptosis in CRC cells not only helps to uncover the mechanisms of drug resistance but also provides useful guidance for improving therapeutic strategies.
The inhibition of cellular ferroptosis in CRC cells may be one of the basics of drug resistance. Lipocalin 2(LCN2) is an iron-trafficking protein that can decrease intracellular iron levels. Meanwhile, LCN2 stimulates the expression of xCT and GPX4. It has been found that LCN2 is highly expressed in various kinds of cancers including CRC, and leads to resistance to 5-fluorouracil (5-FU) in vitro and in vivo by inhibiting ferroptosis.71 Andrographis mitigates resistance to 5FU-based chemotherapy by stimulating ferroptosis and downregulating the β-catenin/Wnt-signaling pathway.72 It was found that KIF20A is overexpressed in oxaliplatin-resistant cell lines and is closely related to patient survival. Silencing KIF20A promotes the ferroptosis process in CRC cells via the KIF20A/NUAK1/PP1β/GPX4 pathway and enhances cellular sensitivity to oxaliplatin.73
RAS mutation is known to be a mechanism of resistance to anti-EGFR antibody therapy in CRC.74 In KRAS mutant CRC cell lines, cetuximab promotes RSL3-induced ferroptosis by activating p38 MAPK and blocking the Nrf2/HO-1 axis.65 β-elemene is extracted from the Chinese herb Curcumae Rhizoma and is known as a bioactive component with broad-spectrum anticancer effects. It was found to induce ferroptosis in CRC cells. By triggering ferroptosis and preventing epithelial–mesenchymal transition, cetuximab and β-elemene together increase the sensitivity of KRAS mutant CRC cells.75 Vitamin C (VitC) could disrupt iron homeostasis and induce ROS-mediated stress ultimately leading to ferroptosis. The combination of VitC and Cetuximab inhibits the growth of CRC cells and prevents the development of acquired resistance to anti-EGFR targeted therapy in vitro.76
Novel Ferroptosis Inducers with Anti-Tumor Activity
Except for the commonly used ferroptosis inducers such as erastin and RSL3, more chemicals and synthesized drugs were found to induce ferroptosis. Cisplatin was reported to trigger ferroptosis in CRC cells achieved by decreasing reduced glutathione as well as guiding the inactivation of glutathione peroxidase. And it can cooperate with erastin to play a synergistic anti-tumor effect.77 Tian et al proved that apatinib stimulated ferroptosis in CRC cells by targeting the elongation of very long-chain fatty acids family member 6 (ELOVL6) and subsequently up-regulating ACSL4 and down-regulating GPX4 and FTH1.78 Dichloroacetate (DCA) can sequester iron in lysosomes, thereby triggering ferroptosis and reducing the stemness of CRC cells.79 2-Imino-6-methoxy-2H-chromene-3-carbothioamide (IMCA) is a benzopyran derivative with broad biological activities and was revealed to induce CRC cells’ ferroptosis in vivo and in vitro. By activating the AMPK/mTOR/p70S6k signaling pathway, IMCA reduces the expression of SLC7A11 and causes ferroptosis.80
Currently, bioactive ingredients from plants and other organisms have attracted wide interest and concern. In addition to the Andrographis mentioned above, bromelain derived from pineapple stem was reported to effectively induce ferroptosis in Kras-mutant CRC cells by regulating ACSL4 levels.81 Punicic acid (PunA, C18:3 c9t11c13) which is present at up to 83% in pomegranate seed oil was known to exert a strong anti-cancer activity. Recent work has presented that PunA triggered intense lipid peroxidation leading to ferroptosis in CRC cells.82 Talaroconvolutin A (talaA) is a natural product isolated from the Talaromyces purpureogenus. It was found to trigger ferroptosis more effectively than erastin by raising ROS levels and decreasing the expression of SLC7A11. Experiments showed that TalaA could significantly inhibit the growth of CRC cells while it did not cause obvious liver and kidney toxicities, which made it a promising drug for CRC therapy.83 Similarly, Auriculasin, a polyphenolic flavonoid isolated from Flemingia philippinensis, promotes ferroptosis, apoptosis, and oxeiptosis in CRC cells by stimulating ROS production, thus inhibiting cell viability, colony formation, and invasion.84
Combination of Ferroptosis Inducers and CRC Treatment
For better therapeutic effects, we highlight the importance of combining ferroptosis with other knowledge areas and techniques. The tumor microenvironment (TME) has significant implications for tumor development, invasion, metastasis, and immune evasion. Immune cells in TME may act as promoting or suppressing factors in tumor growth.85 Ferroptosis also interacts with TME and plays a dual role in cancer immunity.86 Iron and immunity are also closely related. Nowadays, immunotherapy that targets immunological checkpoints and immune microenvironment control has exhibited substantial efficiency in the treatment of cancer.87 Besides, nano-technology is playing a significant role in precision therapy, and various nanomaterials and nanomedicines have been used for the detection and treatment of diseases.
A study by Zhu et al found that myeloid-derived suppressor cells (MDSCs) from colon cancer patients overexpressed asah2, which protected MDSCs from ferroptosis by repressing the p53–Hmox1 axis. And they developed an Asah2-selective small molecular inhibitor, NC06, which can suppress MDSCs accumulation by inducing ferroptosis and activating T cell infiltration to suppress tumor growth in vivo. NC06 has been shown to suppress CRC and some other cancers in preclinical mouse models and may provide greater value in cancer immunotherapy.88
BEBT-908, a dual inhibitor that targets both PI3K and HDAC signaling pathways, effectively induces immunogenic cellular ferroptosis and suppresses tumor growth, thus enhancing immune checkpoint blockade therapy.89
Dihydroartemisinin (DHA) has shown cytotoxicity in various cancers by inducing ferroptosis and would induce more ROS production to cause tumor inhibition in cooperating with an exogenous iron delivery. [email protected]/Pyro-Fe core-shell nanoparticles stabilize DHA against hydrolysis and enhance uptake in tumors, the combination also sensitizes non-immunogenic colorectal tumors to anti-programmed cell death-Ligand 1 (anti-PD-L1) checkpoint blockade immunotherapy.90 The combination of glycyrrhetinic acid-based nanomaterials and ferumoxytol could synergistically increase Fe-dependent cytotoxicity via the Fenton reaction and could synergize PD-L1 blockade to enhance the T-cell immune response against CRC.91
Moreover, conventional treatment of SN38 in combination with electroporation therapy showed better efficacy in CRC therapy. SN38 is the active metabolite of irinotecan, which has become one of the most widely used strategies in first- and second-line treatment of advanced CRC. Such combination changed the redox homeostasis, causing increased generation of intracellular superoxide and depletion of GSH, which may lead to cell death by ferroptosis.92 Electro-assisted sensitization of CRC cells to SN38 opens a new door for innovative CRC treatment.
Conclusion
During the past ten years since ferroptosis was found, there has been remarkable progress in the study of ferroptosis. We gradually shed light on the mechanisms and signal pathways involved in ferroptosis and try to link it with diverse diseases to overcome the therapeutic obstacles. As for CRC, considerable progress has been made but what we saw is just the tip of the iceberg. There are still continuous explorations to reveal the underlying mechanisms and the whole signal pathways. The term that specific lipids undergo oxidation drives the cell death process and results in ferroptosis can be summarized as an imbalance of intracellular oxidative and antioxidant systems. The treatment of cancer has always been regarded as a major challenge in the medical field. As one of the cancers with the greatest incidence and highest mortality rate, CRC urgently needs more effective treatment approaches to improve the prognosis of patients. The particular access by which CRC cells acquire iron from the intestinal lumen and the growth-promoting role of iron in cancer provide more possibilities for the connection between CRC and ferroptosis. Following this way, we have found that specific genes, noncoding RNAs, and proteins showed their potential in regulating ferroptosis in CRC cells and can be developed as therapeutic targets. We give a brief overview of the regulators of ferroptosis and their mechanisms in CRC. Table 1 Regulators of ferroptosis and brief mechanisms in CRC Recent studies have also shown the great therapeutic potential of ferroptosis combined with conventional treatments such as radiotherapy, chemotherapy, and immunotherapy. The development of new drugs, the application of nanotechnology, and the assistance of bioinformatics also provide more power for the treatment of CRC. As a relatively young field, the mechanisms of ferroptosis also intersect with other cell death forms such as apoptosis and autophagy. Iron as well as ferroptotic cells may also have impacts on the tumor microenvironment and, ultimately, on the tumor behavior.93 In the future, the execution mechanisms and diverse triggers of ferroptosis need to be identified to selectively and more effectively control ferroptosis and translate it into therapies. We also attach importance to the potential connections with other cell death forms and tumor microenvironment, and the complex network may be a key point to fight against cancer. Above all, the discovery of ferroptosis blaze a new trial for CRC therapy.
 |
Table 1 Regulators of Ferroptosis and Brief Mechanisms in CRC |
Funding
This study was supported by grants from the National Science Foundation of China (81974227 to YGZ).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi:10.3322/caac.21708
2. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer–analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med. 2000;343(2):78–85. doi:10.1056/nejm200007133430201
3. Song M, Garrett WS, Chan AT. Nutrients, foods, and colorectal cancer prevention. Gastroenterology. 2015;148(6):1244–60.e16. doi:10.1053/j.gastro.2014.12.035
4. Patel SG, Karlitz JJ, Yen T, Lieu CH, Boland CR. The rising tide of early-onset colorectal cancer: a comprehensive review of epidemiology, clinical features, biology, risk factors, prevention, and early detection. Lancet Gastroenterol Hepatol. 2022;7(3):262–274. doi:10.1016/S2468-1253(21)00426-X
5. Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. CELL. 2012;149(5):1060–1072. doi:10.1016/j.cell.2012.03.042
6. Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ. 2018;25(3):486–541. doi:10.1038/s41418-017-0012-4
7. Bebber CM, Müller F, Prieto Clemente L, Weber J, von Karstedt S. Ferroptosis in Cancer Cell Biology. Cancers. 2020;12(1):Jan. doi:10.3390/cancers12010164
8. Riegman M, Sagie L, Galed C, et al. Ferroptosis occurs through an osmotic mechanism and propagates independently of cell rupture. Nat Cell Biol. 2020;22(9):1042–1048. doi:10.1038/s41556-020-0565-1
9. Drakesmith H, Nemeth E, Ganz T. Ironing out Ferroportin. Cell Metab. 2015;22(5):777–787. doi:10.1016/j.cmet.2015.09.006
10. Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi:10.1038/nature13148
11. Chen X, Yu C, Kang R, Tang D. Iron Metabolism in Ferroptosis. Front Cell Dev Biol. 2020;8:590226. doi:10.3389/fcell.2020.590226
12. Sabharwal SS, Schumacker PT. Mitochondrial ROS in cancer: initiators, amplifiers or an Achilles’ heel? Nat Rev Cancer. 2014;14(11):709–721. doi:10.1038/nrc3803
13. Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim Biophys Acta. 2002;1603(1):31–46. doi:10.1016/s0304-419x(02)
14. Ashmore JH, Rogers CJ, Kelleher SL, Lesko SM, Hartman TJ. Dietary iron and colorectal cancer risk: a review of human population studies. Crit Rev Food Sci Nutr. 2016;56(6):1012–1020. doi:10.1080/10408398.2012.749208
15. Yuan Y, Ni S, Zhuge A, Li B, Li L. Iron Regulates the Warburg Effect and Ferroptosis in Colorectal Cancer. Front Oncol. 2021;11:614778. doi:10.3389/fonc.2021.614778
16. Yang WS, Stockwell BR. Ferroptosis: death by Lipid Peroxidation. Trends Cell Biol. 2016;26(3):165–176. doi:10.1016/j.tcb.2015.10.014
17. Yin H, Xu L, Porter NA. Free radical lipid peroxidation: mechanisms and analysis. Chem Rev. 2011;111(10):5944–5972. doi:10.1021/cr200084z
18. Doll S, Proneth B, Tyurina YY, et al. ACSL4 dictates ferroptosis sensitivity by shaping cellular lipid composition. Nat Chem Biol. 2017;13(1):91–98. doi:10.1038/nchembio.2239
19. Kuhn H, Banthiya S, van Leyen K. Mammalian lipoxygenases and their biological relevance. Biochim Biophys Acta. 2015;1851(4):308–330. doi:10.1016/j.bbalip.2014.10.002
20. Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi:10.1016/j.cell.2013.12.010
21. Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi:10.1016/0891-5849(91)
22. Dixon SJ, Patel DN, Welsch M, et al. Pharmacological inhibition of cystine-glutamate exchange induces endoplasmic reticulum stress and ferroptosis. eLife. 2014;3:e02523. doi:10.7554/eLife.02523
23. Xu X, Zhang X, Wei C, et al. Targeting SLC7A11 specifically suppresses the progression of colorectal cancer stem cells via inducing ferroptosis. Eur J Pharm Sci. 2020;152:105450. doi:10.1016/j.ejps.2020.105450
24. Sui X, Zhang R, Liu S, et al. RSL3 Drives Ferroptosis Through GPX4 Inactivation and ROS Production in Colorectal Cancer. Front Pharmacol. 2018;9:1371. doi:10.3389/fphar.2018.01371
25. Bersuker K, Hendricks JM, Li Z, et al. The CoQ oxidoreductase FSP1 acts parallel to GPX4 to inhibit ferroptosis. Nature. 2019;575(7784):688–692. doi:10.1038/s41586-019-1705-2
26. Doll S, Freitas FP, Shah R, et al. FSP1 is a glutathione-independent ferroptosis suppressor. Nature. 2019;575(7784):693–698. doi:10.1038/s41586-019-1707-0
27. Kraft VAN, Bezjian CT, Pfeiffer S, et al. GTP Cyclohydrolase 1/Tetrahydrobiopterin Counteract Ferroptosis through Lipid Remodeling. ACS Cent Sci. 2020;6(1):41–53. doi:10.1021/acscentsci.9b01063
28. Hu Q, Wei W, Wu D, et al. Blockade of GCH1/BH4 axis activates ferritinophagy to mitigate the resistance of colorectal cancer to erastin-induced ferroptosis. Front Cell Dev Biol. 2022;10:810327. doi:10.3389/fcell.2022.810327
29. Pedrera L, Espiritu RA, Ros U, et al. Ferroptotic pores induce Ca(2+) fluxes and ESCRT-III activation to modulate cell death kinetics. Cell Death Differ. 2021;28(5):1644–1657. doi:10.1038/s41418-020-00691-x
30. Wang R, Su Q, Yin H, Wu D, Lv C, Yan Z. Inhibition of SRSF9 enhances the sensitivity of colorectal cancer to erastin-induced ferroptosis by reducing glutathione peroxidase 4 expression. Int J Biochem Cell Biol. 2021;134:105948. doi:10.1016/j.biocel.2021.105948
31. Liu XS, Yang JW, Zeng J, et al. SLC2A1 is a diagnostic biomarker involved in immune infiltration of colorectal cancer and associated with m6A Modification and ceRNA. Front Cell Dev Biol. 2022;10:853596. doi:10.3389/fcell.2022.853596
32. Peng B, Peng J, Kang F, Zhang W, Peng E, He Q. Ferroptosis-Related Gene MT1G as a Novel Biomarker Correlated With Prognosis and Immune Infiltration in Colorectal Cancer. Front Cell Dev Biol. 2022;10:881447. doi:10.3389/fcell.2022.881447
33. Zhong Y, Zhang W, Yu H, et al. Multi-platform-based characterization of ferroptosis in human colorectal cancer. iScience. 2022;25(8):104750. doi:10.1016/j.isci.2022.104750
34. Du S, Zeng F, Sun H, et al. Prognostic and therapeutic significance of a novel ferroptosis related signature in colorectal cancer patients. Bioengineered. 2022;13(2):2498–2512. doi:10.1080/21655979.2021.2017627
35. Liu Y, Guo F, Guo W, Wang Y, Song W, Fu T. Ferroptosis-related genes are potential prognostic molecular markers for patients with colorectal cancer. Clin Exp Med. 2021;21(3):467–477. doi:10.1007/s10238-021-00697-w
36. Shao Y, Jia H, Huang L, et al. An original ferroptosis-related gene signature effectively predicts the prognosis and clinical status for colorectal cancer patients. Front Oncol. 2021;11:711776. doi:10.3389/fonc.2021.711776
37. Wang Y, Xia HB, Chen ZM, Meng L, Xu AM. Identification of a ferroptosis-related gene signature predictive model in colon cancer. World J Surg Oncol. 2021;19(1):135. doi:10.1186/s12957-021-02244-z
38. Yang C, Huang S, Cao F, Zheng Y. Role of ferroptosis-related genes in prognostic prediction and tumor immune microenvironment in colorectal carcinoma. PeerJ. 2021;9:e11745. doi:10.7717/peerj.11745
39. Cheok CF, Verma CS, Baselga J, Lane DP. Translating p53 into the clinic. Nat Rev Clin Oncol. 2011;8(1):25–37. doi:10.1038/nrclinonc.2010.174
40. Ji H, Wang W, Li X, et al. p53: a double-edged sword in tumor ferroptosis. Pharmacol Res. 2022;177:106013. doi:10.1016/j.phrs.2021.106013
41. Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi:10.1038/nature14344
42. Xie Y, Zhu S, Song X, et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017;20(7):1692–1704. doi:10.1016/j.celrep.2017.07.055
43. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
44. Muers M. RNA: genome-wide views of long non-coding RNAs. Nat Rev Genet. 2011;12(11):742. doi:10.1038/nrg3088
45. Arun G, Diermeier SD, Spector DL. Therapeutic Targeting of Long Non-Coding RNAs in Cancer. Trends Mol Med. 2018;24(3):257–277. doi:10.1016/j.molmed.2018.01.001
46. Tay Y, Rinn J, Pandolfi PP. The multilayered complexity of ceRNA crosstalk and competition. NATURE. 2014;505(7483):344–352. doi:10.1038/nature12986
47. Luo Y, Huang S, Wei J, et al. Long noncoding RNA LINC01606 protects colon cancer cells from ferroptotic cell death and promotes stemness by SCD1-Wnt/β-catenin-TFE3 feedback loop signalling. Clin Transl Med. 2022;12(4):e752. doi:10.1002/ctm2.752
48. Zeng JH, Liang L, He RQ, et al. Comprehensive investigation of a novel differentially expressed lncRNA expression profile signature to assess the survival of patients with colorectal adenocarcinoma. Oncotarget. 2017;8(10):16811–16828. doi:10.18632/oncotarget.15161
49. Zhang W, Fang D, Li S, Bao X, Jiang L, Sun X. Construction and validation of a novel ferroptosis-related lncrna signature to predict prognosis in colorectal cancer patients. Front Genet. 2021;12:709329. doi:10.3389/fgene.2021.709329
50. Liu L, Yao H, Zhou X, et al. MiR-15a-3p regulates ferroptosis via targeting glutathione peroxidase GPX4 in colorectal cancer. Mol Carcinog. 2022;61(3):301–310. doi:10.1002/mc.23367
51. Yang Y, Lin Z, Han Z, et al. miR-539 activates the SAPK/JNK signaling pathway to promote ferroptosis in colorectal cancer by directly targeting TIPE. Cell Death Discov. 2021;7(1):272. doi:10.1038/s41420-021-00659-x
52. Zheng S, Hu L, Song Q, et al. miR-545 promotes colorectal cancer by inhibiting transferring in the non-normal ferroptosis signaling. Aging. 2021;13(24):26137–26147. doi:10.18632/aging.203801
53. Angius A, Uva P, Pira G, et al. Integrated Analysis of miRNA and mRNA Endorses a Twenty miRNAs Signature for Colorectal Carcinoma. Int J Mol Sci. 2019;20(16):4067. doi:10.3390/ijms20164067
54. Zhong Y, Du Y, Yang X, et al. Circular RNAs function as ceRNAs to regulate and control human cancer progression. Mol Cancer. 2018;17(1):79. doi:10.1186/s12943-018-0827-8
55. Yang Z, Xie L, Han L, et al. Circular RNAs: regulators of Cancer-Related Signaling Pathways and Potential Diagnostic Biomarkers for Human Cancers. Theranostics. 2017;7(12):3106–3117. doi:10.7150/thno.19016
56. Wang Y, Chen H, Wei X. Circ_0007142 downregulates miR-874-3p-mediated GDPD5 on colorectal cancer cells. Eur J Clin Invest. 2021;51(7):e13541. doi:10.1111/eci.13541
57. Xian ZY, Hu B, Wang T, et al. CircABCB10 silencing inhibits the cell ferroptosis and apoptosis by regulating the miR-326/CCL5 axis in rectal cancer. Neoplasma. 2020;67(5):1063–1073. doi:10.4149/neo_2020_191024N1084
58. Kobayashi A, Kang MI, Okawa H, et al. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol Cell Biol. 2004;24(16):7130–7139. doi:10.1128/mcb.24.16.7130-7139.2004
59. Kobayashi A, Kang MI, Watai Y, et al. Oxidative and electrophilic stresses activate Nrf2 through inhibition of ubiquitination activity of Keap1. Mol Cell Biol. 2006;26(1):221–229. doi:10.1128/mcb.26.1.221-229.2006
60. Ishii T, Itoh K, Takahashi S, et al. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem. 2000;275(21):16023–16029. doi:10.1074/jbc.275.21.16023
61. Wakabayashi N, Itoh K, Wakabayashi J, et al. Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet. 2003;35(3):238–245. doi:10.1038/ng1248
62. Fan Z, Wirth AK, Chen D, et al. Nrf2-Keap1 pathway promotes cell proliferation and diminishes ferroptosis. Oncogenesis. 2017;6(8):e371. doi:10.1038/oncsis.2017.65
63. Sun X, Ou Z, Chen R, et al. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63(1):173–184. doi:10.1002/hep.28251
64. Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6(27):24393–24403. doi:10.18632/oncotarget.5162
65. Yang J, Mo J, Dai J, et al. Cetuximab promotes RSL3-induced ferroptosis by suppressing the Nrf2/HO-1 signalling pathway in KRAS mutant colorectal cancer. Cell Death Dis. 2021;12(11):1079. doi:10.1038/s41419-021-04367-3
66. Wei R, Zhao Y, Wang J, et al. Tagitinin C induces ferroptosis through PERK-Nrf2-HO-1 signaling pathway in colorectal cancer cells. Int J Biol Sci. 2021;17(11):2703–2717. doi:10.7150/ijbs.59404
67. Malfa GA, Tomasello B, Acquaviva R, et al. Betula etnensis Raf. (Betulaceae) Extract Induced HO-1 Expression and Ferroptosis Cell Death in Human Colon Cancer Cells. Int J Mol Sci. 2019;20(11). doi:10.3390/ijms20112723
68. Singhal R, Mitta SR, Das NK, et al. HIF-2α activation potentiates oxidative cell death in colorectal cancers by increasing cellular iron. J Clin Invest. 2021;131(12). doi:10.1172/jci143691
69. Song J, Liu T, Yin Y, et al. The deubiquitinase OTUD1 enhances iron transport and potentiates host antitumor immunity. EMBO Rep. 2021;22(2):e51162. doi:10.15252/embr.202051162
70. Hong SH, Lee DH, Lee YS, et al. Molecular crosstalk between ferroptosis and apoptosis: emerging role of ER stress-induced p53-independent PUMA expression. Oncotarget. 2017;8(70):115164–115178. doi:10.18632/oncotarget.23046
71. Chaudhary N, Choudhary BS, Shah SG, et al. Lipocalin 2 expression promotes tumor progression and therapy resistance by inhibiting ferroptosis in colorectal cancer. Int J Cancer. 2021;149(7):1495–1511. doi:10.1002/ijc.33711
72. Sharma P, Shimura T, Banwait JK, Goel A. Andrographis-mediated chemosensitization through activation of ferroptosis and suppression of β-catenin/Wnt-signaling pathways in colorectal cancer. Carcinogenesis. 2020;41(10):1385–1394. doi:10.1093/carcin/bgaa090
73. Yang C, Zhang Y, Lin S, Liu Y, Li W. Suppressing the KIF20A/NUAK1/Nrf2/GPX4 signaling pathway induces ferroptosis and enhances the sensitivity of colorectal cancer to oxaliplatin. Aging. 2021;13(10):13515–13534. doi:10.18632/aging.202774
74. Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. 2014;4(11):1269–1280. doi:10.1158/2159-8290.Cd-14-0462
75. Chen P, Li X, Zhang R, et al. Combinative treatment of β-elemene and cetuximab is sensitive to KRAS mutant colorectal cancer cells by inducing ferroptosis and inhibiting epithelial-mesenchymal transformation. Theranostics. 2020;10(11):5107–5119. doi:10.7150/thno.44705
76. Lorenzato A, Magrì A, Matafora V, et al. Vitamin C Restricts the Emergence of Acquired Resistance to EGFR-Targeted Therapies in Colorectal Cancer. Cancers. 2020;12(3):Mar. doi:10.3390/cancers12030685
77. Guo J, Xu B, Han Q, et al. Ferroptosis: a Novel Anti-tumor Action for Cisplatin. Cancer Res Treat. 2018;50(2):445–460. doi:10.4143/crt.2016.572
78. Tian X, Li S, Ge G. Apatinib Promotes Ferroptosis in Colorectal Cancer Cells by Targeting ELOVL6/ACSL4 Signaling. Cancer Manag Res. 2021;13:1333–1342. doi:10.2147/cmar.S274631
79. Sun J, Cheng X, Pan S, et al. Dichloroacetate attenuates the stemness of colorectal cancer cells via triggering ferroptosis through sequestering iron in lysosomes. Environ Toxicol. 2021;36(4):520–529. doi:10.1002/tox.23057
80. Zhang L, Liu W, Liu F, et al. IMCA Induces Ferroptosis Mediated by SLC7A11 through the AMPK/mTOR Pathway in Colorectal Cancer. Oxid Med Cell Longev. 2020;2020:1675613. doi:10.1155/2020/1675613
81. Park S, Oh J, Kim M, Jin EJ. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim Cells Syst. 2018;22(5):334–340. doi:10.1080/19768354.2018.1512521
82. Vermonden P, Vancoppenolle M, Dierge E, et al. Punicic acid triggers ferroptotic cell death in carcinoma cells. Nutrients. 2021;13(8):Aug. doi:10.3390/nu13082751
83. Xia Y, Liu S, Li C, et al. Discovery of a novel ferroptosis inducer-talaroconvolutin A-killing colorectal cancer cells in vitro and in vivo. Cell Death Dis. 2020;11(11):988. doi:10.1038/s41419-020-03194-2
84. Wang CX, Chen LH, Zhuang HB, et al. Auriculasin enhances ROS generation to regulate colorectal cancer cell apoptosis, ferroptosis, oxeiptosis, invasion and colony formation. Biochem Biophys Res Commun. 2022;587:99–106. doi:10.1016/j.bbrc.2021.11.101
85. Lei X, Lei Y, Li JK, et al. Immune cells within the tumor microenvironment: biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–133. doi:10.1016/j.canlet.2019.11.009
86. Lei G, Zhuang L, Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat Rev Cancer. 2022;22(7):381–396. doi:10.1038/s41568-022-00459-0
87. Hinshaw DC, Shevde LA. The tumor microenvironment innately modulates cancer progression. Cancer Res. 2019;79(18):4557–4566. doi:10.1158/0008-5472.Can-18-3962
88. Zhu H, Klement JD, Lu C, et al. Asah2 Represses the p53-Hmox1 Axis to Protect Myeloid-Derived Suppressor Cells from Ferroptosis. J Immunol. 2021;206(6):1395–1404. doi:10.4049/jimmunol.2000500
89. Fan F, Liu P, Bao R, et al. A Dual PI3K/HDAC Inhibitor Induces Immunogenic Ferroptosis to Potentiate Cancer Immune Checkpoint Therapy. Cancer Res. 2021;81(24):6233–6245. doi:10.1158/0008-5472.Can-21-1547
90. Han W, Duan X, Ni K, Li Y, Chan C, Lin W. Co-delivery of dihydroartemisinin and pyropheophorbide-iron elicits ferroptosis to potentiate cancer immunotherapy. BIOMATERIALS. 2022;280:121315. doi:10.1016/j.biomaterials.2021.121315
91. Li Q, Su R, Bao X, et al. Glycyrrhetinic acid nanoparticles combined with ferrotherapy for improved cancer immunotherapy. Acta Biomater. 2022. doi:10.1016/j.actbio.2022.03.030
92. Nikolova B, Semkova S, Tsoneva I, et al. Redox-related molecular mechanism of sensitizing colon cancer cells to camptothecin analog SN38. Anticancer Res. 2020;40(9):5159–5170. doi:10.21873/anticanres.14519
93. Wiernicki B, Maschalidi S, Pinney J, et al. Cancer cells dying from ferroptosis impede dendritic cell-mediated anti-tumor immunity. Nat Commun. 2022;13(1):3676. doi:10.1038/s41467-022-31218-2


