Cell-Based Drug Delivery Systems with Innate Homing Capability as a Novel Nanocarrier Platform

Introduction
When many anti-cancer agents are applied in vivo, they are characterized by suboptimal pharmacokinetic performance. Some features include unsatisfactory tumor targeting, low solubility, poor stability, and rapid clearance induced by the mononuclear phagocytic system. Small molecule drugs are also commonly accompanied by numerous adverse effects owing to high toxicity in non-target tissue.1 In the past decades, drug delivery systems (DDSs) have been extensively studied to address these issues.2 The design of the DDS aims to increase the therapeutic dose of the drug at the target site while maintaining sustained drug release. The main functions of nanocarriers include prolonged circulation time and specific tissue targeting.3 Usually, nanoparticles (NPs),4 bacteria,5 and viruses are utilized as delivery vehicles to promote drug stability and transport the drug to the desired site.6 Recently, the development of nanocarriers has largely promoted the advancement of DDS.7 However, nanocarriers have several limitations, such as the poor ability to cross the blood-brain barrier (BBB) and inadequate intratumoral penetration, liver clearance, and immunogenicity. To overcome these limitations, cell-mediated DDSs have emerged as a new frontier in nanomedicine, in which red blood cells (RBCs), neutrophils, macrophages, T cells, and stem cells as drug delivery vehicles due to their dynamic roles in biological systems.8 Compared to conventional DDSs, cell-based systems show prolonged circulation, specific tissue tropism, superior flexibility, low immunogenicity, and cytotoxicity with unique cellular properties. Furthermore, cell-based DDSs are intrinsically biodegradable and biocompatible. The ability of therapeutically relevant cells to home toward injured tissues allows cell-based drug carriers to act as a biomimetic platform and deliver therapeutic agents to specific sites.9 As a result, cell-mediated DDSs are a promising strategy to maximize therapeutic outcomes and minimize the side effects for the drug treatment of various diseases (Table 1).
 |
Table 1 Applications of Cell-Based Drug Delivery Systems |
In this review, we focus on the unique properties of cell-based drug carrier candidates and their homing mechanisms to tumor sites. In addition, we summarize different techniques used to load drugs inside cells or to conjugated them on the cell surface (Figure 1).
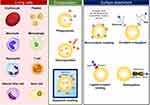 |
Figure 1 Scheme of living cell-based drug delivery systems. |
Cells as Drug Delivery Carriers
Blood Cells
Erythrocytes
Erythrocytes, or RBCs, constitute the majority of blood cells (>99%) and are the main oxygen carriers to tissues via the circulatory system. Erythrocytes, which are developed in the bone marrow and circulate in the human body for ~100–120 days before being recycled by macrophages, can be easily isolated, frozen, and stored for at least ten years. Each complete circulation period is ~60 seconds.10 To save as much space as possible for oxygen, the nuclei and most cellular organelles must be sacrificed in mature erythrocytes. Furthermore, the biconcave shape of RBCs, which have diameters ranging from 6.2 to 8.2 μm, provides a high surface-to-volume ratio for oxygen supply and greater flexibility when traversing the circulatory system, particularly when squeezing through narrow capillary networks.11
In addition to oxygen, erythrocytes transport a variety of valuable payloads, including drugs and imaging contrast agents.12 RBCs offer high drug loading owed to their biconcave shape and non-nuclear structure. Furthermore, the erythrocyte’s membrane has reversible deformability, allowing them to encapsulate payloads using mechanical techniques. In addition, the long half-life of RBCs allows a sustained release of therapeutic material to the vascular system. Since the reticuloendothelial system (RES) recognizes old and incompatible erythrocytes and rapidly eliminates them without producing toxic byproducts, they are completely biodegradable and have superior biocompatibility. Therefore, RBC clearance pathways have been commonly employed to target the RES of the liver, spleen, and bone marrow.13
The attractive properties of RBCs means they have been exploited since 1973 as smart biological carriers to transport and distribute diverse pharmacological agents, such as antiviral (glutathione) and anti-inflammatory agents (dexamethasone 21-phosphate),14,15 antigens,16 nucleic acids (mRNA),17 peptides,18 and enzymes (β-glucosidase and β-galactosidase).19 However, using RBCs as drug delivery vehicles encounters several difficulties. For example, the RES can rapidly remove cellular modified or drug encapsulation RBCs due to morphological and functional changes. In addition, RBCs cannot migrate across the vascular endothelium to release therapeutic agents to injured tissue, which means it is hard to deliver drugs to the site of inflammation, infection, or cancer. Therefore, non-RES targeting with RBC carriers is challenging,20,21 and immune and stem cells are the cell candidate of interest for cell-based drug delivery outside of the circulatory system.
Platelets
Platelets are a type of anucleate blood cell that aggregate around damaged blood vessels to prevent blood leakage. The life span of platelets is about 7–10 days. Therefore, the shorter half-life of platelets, compared to erythrocytes, can help obtain optimal drug concentrations within a shorter time frame.22 Platelets can also send “don’t eat me” signals to macrophages due to their cluster off differentiation (CD) 47 membrane expression, preventing phagocytosis. As a result, platelet-mediated DDSs are expected to promote escape from macrophage-mediated clearance and enhance drug retention time, and, therefore, tumor accumulation.23 Platelets can precisely target specific cells or sites and naturally release their drug cargo faster in cancerous tissue’s acidic environment compared to healthy tissue. Therefore, drug release from platelets is stimuli responsive once they target tumor. As a result of the intrinsic homing properties of platelets to tumor cells, platelet-mediated DDSs can target multiple metastatic lesions in the body.24,25 By loading platelets with anti-cancer drugs, solid tumors are targeted and induced toward tumor regression. Therefore, the characteristics of platelets provide efficient storage, trafficking, tumor targeting, and controlled drug release.
Immune Cells
Immune cells have been identified as an interesting cell-based delivery system due to their intrinsic transport mechanisms. The endothelium wall along the vasculature is a monolayer of cells that controls materials’ permeability across the tissue. The physiological barrier of an interendothelial passage is generally <3 nm; therefore, few drugs are transported across to where they can elicit their mechanism of action at the diseased site.26,27 Initial research has shown that immune cells can actively cross the endothelium barrier, some even penetrating the BBB.28,29 Innate and adaptive immune responses against infection, inflammation, tissue injury, and cancer are featured by the widespread recruitment of millions of immune cells that can rapidly respond by activation, adhesion, and migration across endothelial vessels.30–32 Therefore, drug-loaded immune cells, such as neutrophils, monocytes, macrophages, T cells, and natural killer cells (NK) work as “Trojan horses” for mediating drug transport across the endothelial barrier; drugs can be released to specific disease sites while maintaining superior biocompatibility.
Neutrophils
Neutrophils are the most abundant type of leukocyte, accounting for 40–70% with an approximate half-life of 8 h in humans.31 Neutrophils are the first-in-line defenders against pathogen invasion. During such an event, neutrophils frequently enter the tissue from the bloodstream through the endothelium, a crucial step in innate immunity. Additionally, neutrophils engulf invading foreign substances or microorganisms, removing the invaders using digestive enzymes, such as lysozymes, hydrolytic enzymes, and myeloperoxidase, or respiratory burst.33 Furthermore, with their particular homing ability, neutrophils can be utilized as natural drug carriers to target inflammatory areas. For example, Chu et al demonstrated that activated neutrophils mediated the delivery of drug-loaded albumin nanoparticles across blood vessels to sites of inflammation; this significantly mitigated lipopolysaccharide-induced inflammation or Pseudomonas aeruginosa-induced infection of the lung.34 However, neutrophils have a short lifespan (~5 days) in circulation and only a few hours after being isolated from blood, which limits their use in DDSs. Nonetheless, neutrophils are attractive candidates because of their migration and transport capabilities. Consequently, neutrophil-based DDSs have considerable potential for treating various brain illnesses involving neutrophil infiltration, such as multiple sclerosis,35 Alzheimer’s disease,36 stroke,37,38 and traumatic brain injury.39,40
Monocytes
Monocytes are the largest leukocytes, with a diameter of 50–80 μm, and account for around 2–10% of all leukocytes in human blood. Unlike erythrocytes and platelets, monocytes contain unilobar nuclei and have a granulated cytoplasm. Indeed, monocytes are regarded as the precursors of macrophages and myeloid lineage dendritic cells (DCs) and can phagocytize to eliminate damaged cells.41 Monocytes originate from bone marrow stem cell precursors and differentiate into macrophages once they leave the bloodstream and can respond to diverse stimulants. In contrast, deactivated monocytes return to the bone marrow.42 It has been demonstrated that ligand-conjugated, single-walled carbon nanotubes loaded on monocytes enhanced the tumor-targeting efficiency of monocyte carriers.43
Macrophages
Macrophages are monocyte-derived specialized cells that are part of the innate immune system. They are distinguished by their irregular shape, large size (5–50μm in diameter), and exceptional immunological flexibility. Circulating macrophages typically have a half-life of 1–3 days.44 The ability of these cells to phagocytize bacteria and other particles is their most distinguishable characteristic.45,46 Many efforts have been made to produce macrophages for drug delivery due to their unique properties. First, macrophages are known for their tendency to travel along a chemoattractant gradient toward diseased locations, which are typically sites of inflammation and malignancies.47 Second, macrophages can recognize and effectively eliminate foreign objects traveling in the blood, such as endotoxins,48 polysaccharides,49 and low-density lipoproteins.50 Third, macrophages can target various pathological illnesses, including cancer, viral, and autoimmune diseases.51 Furthermore, macrophages tend to congregate in hypoxic areas. This feature can be manipulated to target anti-cancer agents to areas frequently linked with poor drug delivery.52 Fourth, at the location of the disease site, macrophages are activated by several stimuli, which can cause the release of intracellular substances, such as cytotoxic payloads.53 Consequently, macrophages are appealing drug delivery vehicles.
Monocytes and macrophages can serve as drug carriers to deliver therapies to the brain, as they withhold the capability to cross the BBB. An experimental Parkinson’s disease model demonstrated that bone marrow-derived macrophages loaded with catalase infiltrated the brain and improved the delivery efficiency of catalase two-fold compared to the free or unbound drug, which attenuated neuroinflammation and nigrostriatal degeneration.54 However, concerns remain. For example, off-target effects have not been thoroughly studied, which is essential to understanding the risks of adverse events in the clinical application of macrophages as drug delivery carriers. The ideal macrophage-based drug carrier will either preferentially accumulate at disease sites or selectively exert therapeutic activity at target sites while remaining inert elsewhere.55 Direct loading of a free drug inside macrophages can result in poor drug loading, drug inactivation, and limited drug distribution to target cells due to intracellular degradation.56 As a result, the therapeutic payload must be conjugated to the membrane surface of the macrophages.
T Cells
T cells are derived from pluripotent stem cells in bone marrow. T cells, part of the adaptive immune system, naturally express receptors for a wide range of antigens.57 More specifically, a T cell can express a receptor for a particular antigen of interest, making T cells outstanding for targeting cells. When a cytotoxic T cell encounters its antigen, the T cell receptor (TCR) increases the cell surface reduction potential, prompting the cell to secrete proteins that cause the antigen-presenting cell to die.58 Their high cell specificity and triggering apoptosis make T cells attractive drug delivery carriers to target and eliminate malignancies. In addition to inducing tumor cell apoptosis, activated T cells can secrete cytokines, including interleukin (IL)-2, IL-6, interferon-gamma, and granulocyte-macrophage colony-stimulating factor, to recruit and activate immune cells, such as macrophages and NK cells; this enhances anti-inflammatory and antitumor effects.59,60
However, since the immune activity of T cells against tumors is frequently suppressed during tumor progression, engineered T cell immunotherapies, such as TCR and chimeric antigen receptor (CAR)-engineered T cell therapies, have been developed.61 The key to CAR-T and TCR-T cell therapies is activating T cells to kill tumor cells; this is achieved through gene editing to express CARs or TCRs on the T cell surface that detect tumor cells. Therefore, T cells modified with functional receptors are a subtype of cell-based DDSs in which the loaded therapeutic agent is the genetic material that can express specific molecules with therapeutic effects. To optimize intratumoral distribution of activated T cells, an adenosine antagonist loaded within multilamellar liposomal vesicles were crosslinked to CAR-T cells to inhibit inactivation of tumor-infiltrating T cells deeply within the immunosuppressive tumor microenvironment (TME).62–64
Natural Killer Cells (NK)
NK cells are large granular lymphocytes that contribute to the innate immune system, accounting for 5–10% of circulating lymphocytes. In contrast to T and B cells, NK cells can kill tumor cells rapidly without recognizing tumor-specific antigens, which means they have enormous potential in cancer immunotherapy.65,66 To cause direct cytotoxicity, NK cells release perforins, tumor necrosis factor (TNF), and granzymes. Alternatively, NK cells can induce tumor cell apoptosis via Fas/Fas-ligand interactions.67,68 NK cells can also activate macrophages and DCs by the production of chemokines and cytokines,69 inducing antitumor immunity. Deng et al encapsulated photodynamic agents in NK cells and demonstrated that NK cell-based DDS provided a strong immunotherapeutic effect during the antitumor process.70
However, tumor cells can escape or inhibit NK cell activity by reducing the expression of Fas and producing immunosuppressive cytokines.71 Therefore, CAR-modified NK cells have emerged as an improved NK cell-mediated tumor immunotherapy strategy. Zhang et al modified NK cells to express the erythroblastic oncogene (Erb)B2-specific CAR and identified that these cells had specific cytotoxicity to ErbB2-expressed glioma.72 Even though NK cell-mediated DDSs are promising in chemotherapy and immunotherapy, there are critical limitations to overcome, such as NK cells’ difficulty passing through physical and biological barriers to reach the tumor parenchyma, which frequently results in unsatisfactory therapeutic outcomes.73
Stem Cells
Stem cells are multipotent cells that have self-renewal and self-replication capabilities. They are classified into two categories based on origin and plasticity, embryonic and adult stem cells. Embryonic stem cells are pluripotent cells that can differentiate into any cell type and are derived from the inner cell mass of a blastocyst in an early-stage embryo. Adult stem cells, such as mesenchymal stem cells (MSCs), neural stem cells (NSCs), and hematopoietic stem cells, are tissue-specific stem cells with the ability to differentiate into a limited number of specialized cells.74,75 The multi-directional differentiation and immunomodulatory capabilities are why stem cells have widely been used in the fields of regenerative medicine and tissue engineering.76–78
Stem cells have received attention as promising candidates for cell-based drug delivery because they can survive in cancerous environments and tolerate chemotherapeutic agents. Stem cells are also biodegradable and biocompatible. Moreover, they have regenerative, anti-inflammatory,79 and immunomodulatory characteristics.80 The inherent tumor tropism of stem cells is driven by tumor-derived and inflammatory factors and tumor-specific receptors.81–83 The migration of stem cells to tumor sites can occur in 2–4 days. Additionally, stem cells are relatively simple to isolate and culture in vitro; under specific conditions, they can be differentiated into various specialized cells. In addition to being effective drug carriers, stem cells offer effective therapeutic effects with the trophic factors they secrete.84 As a result, stem cells have mainly been employed to regenerate diseased tissues and to fight cancers, such as lung adenocarcinoma (docetaxel),85 glioblastoma (paclitaxel),86 and leukemia.87 Even so, using stem cells as drug carriers involves obstacles that must be overcome, including infection, immunogenicity, tumorigenicity, poor retention after transplantation, and low drug-loading efficiency.88
Homing Ability of Cells
Once cell-based drug carriers are delivered in vivo, the complexes can be dispersed in the bloodstream and navigated to the desired target sites. The intrinsic capability of cell-based carriers to deliver therapeutic payloads to specific tissues in a controlled manner can help optimize therapeutic outcomes compared to dosing the free drug. However, various microenvironments can affect the biodistribution of cell therapies. Within different microenvironments, different proteins are secreted and can induce a range of altering signal cascades that will govern the behavior of cell-based DDSs. Receptors for chemokines and growth factors are abundantly expressed on the surface of cell-based DDSs. The homing process includes the participation of chemokines,89 growth factors,90 adhesion molecules,91 enzymes, and other ligands.92 Therefore, receptor expression on the cell carrier assists in driving the homing pathway. Consequently, this section discusses the mechanism and associated molecules for tumor tropism and homing related to hypoxic tumor microenvironments.
Homing Mechanism
Many circulating cells, such as neutrophils, monocytes/macrophages, and stem cells, can target tumors, wounds, and ischemia regions; this is the most crucial reason for developing cell-based DDSs. For example, tumor-homing, one of the most studied homing mechanisms of living cells, suggests a variety of cells can track circulating cancer cells in the blood and sense primary tumors and metastatic tumor regions.93 The homing mechanism for leukocytes includes the following steps: tethering, rolling, adhesion, crawling, and finally, transmigration (Figure 2).
 |
Figure 2 Mechanism of neutrophil tumor homing. |
Neutrophils are the first cells recruited by chemoattractants, cytokines, such as TNF-α and IL-1β, growth factors, and bacterial products.94–96 Increasing the expression of P-selectin and E-selectin adhesion molecules promotes neutrophil recruitment.31 These selectins bind to their glycosylated ligands, such as P-selectin glycoprotein ligand 1, once they reach the endothelium surface, tethering free-flowing neutrophils to the endothelium and promoting their subsequent rolling along the vessel in the direction of blood flow.97,98 This process favors the transmigration of neutrophils as the cells interact with chemokines and cell adhesion molecules (CAMs) throughout the chemokine gradient along the endothelium, thus prompting firm attachment to the endothelial wall.99,100 Finally, to leave the vasculature, neutrophils cross the endothelium, basement membrane, and pericytes to arrive at disease sites; this requires integrins, CAMs, and different junctional proteins.96,98 Similarly, other immune cells are recruited by chemokines, cytokines, and growth factors, following an immune response.98,101,102
Stem cells naturally home towards injured tissues due to highly expressed chemokine receptors, such as C-C chemokine receptor 2 and C-X-C chemokine receptor 4 (CXCR4), on their cell surfaces.103,104 Therefore, stem cells exhibit tumor tropism and capabilities to infiltrate the BBB.105,106 In addition, the homing mechanism of stem cells contains a memory function; stem cells can remember the injury site and store the memory of the related trauma or inflammation.107,108
Hypoxic Tumor Microenvironment
Tumors behave similarly to wounds that do not heal by working to sustain the microenvironment of the damaged tissue. The microenvironments that tumors promote include hypoxia, mechanical stress, elevated oxidative or nitrosative stress, and sustained inflammation (Figure 3).109,110 The production of pro-angiogenic molecules is a common feature between inflammation and hypoxic conditions. The hypoxia-induced transcription factor (HIF) stimulates the transcription of genes, such as vascular endothelial growth factor, macrophage migration inhibitory factor, TNF-α, nuclear factor kappa B (NF-κB), and several other proinflammatory cytokines.111–113 NF-κB is commonly activated in response to inflammatory mediators and has been demonstrated to induce numerous chemokines, including regulated upon activation, normal T cell expressed and presumably secreted (RANTES) (C-C chemokine ligand [CCL]5), macrophage inflammatory protein (MIP)-2 (C-X-C chemokine ligand 2 [CXCL2]), MIP-1 (CCL3), monocyte chemoattractant protein 1 (MCP-1) (CCL2), and IL-8 (CXCL8), involved in leukocyte migration. Particularly, MCP-1 (CCL2) and RANTES (CCL5) attract and activate mononuclear macrophages.114,115
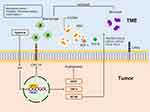 |
Figure 3 Mechanisms of hypoxic tumor microenvironment. |
Cytokines also impact the migration of monocytes to tumors. Colony stimulating factor-1 (CSF-1), the main cytokine, is produced by monocytes, macrophages, and other cells, and affects cell growth, differentiation and the survival of mononuclear phagocytes. In human tumors, augmented expression of CSF-1 and its receptor (CSF-1R) attracts and recruits macrophages to tumors.116 Tumor-derived factors stimulate the differentiation of tumor-infiltrating monocytic myeloid-derived suppressor cells into immunosuppressive tumor-associated macrophages. CSF-1 is the primary mediator of this conversion.117,118
In a diseased environment, cytokines (stromal cell-derived factor 1 [SDF-1]), growth factors (transforming growth factor beta [TGF-β]), and adhesion factors (CAMs) are produced and participate in MSC homing.119,120 Because chemokine and CXC receptors are abundantly expressed on the surface of stem cells, interaction of the MSCs with chemokines drives homing of MSCs to the locations of inflammation. Crosstalk between SDF-1 (CXCL12) and the native CXCR4 receptor of stem cells controls homing considerably, and receptor-ligand response is essential to mediate stem cell tropism.121
Hypoxia in the tumor microenvironment is a cyclic event that perpetuates the inflammatory response by assuring the continuous release of angiogenic and inflammatory mediators. Therefore, hypoxia produces chemokines that regulate the migration of immune cells, and possibly MSCs, to tumors.
Drug Loading Methods
Essential requirements for drugs or drug-loaded carriers are negligible cytotoxicity, degradability, and controlled drug release. Therefore, drug and particle-cell complexes must have robust binding to the target tissue, prolonged circulation time, and minimal immunogenicity. Currently, methods to modify cells for therapeutic purposes can be categorized into two: encapsulating therapeutic agents inside cells or attaching them to the cell membrane (Figure 4). Cells comprise biomolecules with various functional groups, such as proteins, lipids, and polysaccharides, that can form small molecule drug conjugates. Alternatively, the properties of cell membranes can aid in drug encapsulation, forming drug-cell or drug-loaded particle-cell complexes for cell-mediated drug delivery.
Encapsulation
Therapeutic agents can be loaded into cells in various ways. However, for in vivo applications, intracellular drug loading is the most widely used method as drugs are separated from the internal environment, decreasing toxic side effects and extending the half-life. The three techniques for intracellular drug loading that will be described are phagocytosis, electroporation, and hypotonic swelling.
Phagocytosis
Endocytosis is an innate biological mechanism occurring when eukaryotic cells engulf fluid, chemicals, or other cells. Most substances, particularly large and polar molecules, are difficult to pass directly through hydrophobic cell membranes. Endocytosis allows these substances to be internalized via plasma membrane deformation.122 During this process, intracellular vesicles are formed around the internalized substances through plasma membrane invagination, followed by membrane fusion.123
Phagocytosis is a mode of endocytosis that occurs sporadically in specialized cells, such as neutrophils, macrophages, lymphocytes, and DCs. For example, when stimulated by immune responses, leukocytes can internalize nano or micro-sized particles by an actin-dependent mechanism related to receptor reorganizations, such as fragment crystallizable receptors, complement factors, and mannoses.124,125 Therefore, phagocytosis is an immune defense mechanism against foreign invaders and is gaining much attention in cell-mediated DDS design.126
Phagocytosis is a powerful pathway to load therapeutic agents. Drug-loaded particles are prone to rapid RES clearance in vivo, and immune cells are the first to detect and phagocytose particles. Rather than designing particles to withstand swift RES removal, phagocytic cells can be used as delivery vehicles.127 Successful loading of therapeutic cargos inside cell-based vehicles depends on various factors, such as size, shape, surface charge, and composition of the drug carrier.128,129 However, precisely managing cell internalization remains a substantial challenge due to its complexity; it involves various receptors and a succession of fusion and fission events. Therefore, to improve phagocytic drug loading into cell-based DDSs, new strategies for fine-tuning particle characteristics and phagocytosis processes are required.130
Electroporation
Electroporation is a physical method that creates temporary pores on the surface of cells by using an electrical pulse. Cells are typically suspended in a conductive fluid allowing a high-intensity external electrical field to be used. The disruption of the phospholipid bilayer can induce temporary malfunction of the cell membrane, resulting in the encapsulation of foreign substances in the cells. In this procedure, the size of the engulfed molecule determines whether it may enter the cell successfully. The cell keeps its morphology if the engulfed molecules are larger than the pore size; otherwise, the cells may swell, and its membrane may rupture.131,132 Various molecules, such as enzymes (alcohol dehydrogenase),133 nucleic acids (antisense oligodeoxynucleotides),134 and anionic medicates, have been encapsulated in RBCs with sustained-release and non-phagocytic effects.135
Electroporation is also an effective approach to deliver messenger RNA or small interfering RNA into monocytes to serve as antitumor vaccines.17,136,137 However, electroporation has certain drawbacks, including cell membrane damage and partially irreversible structural integrity degradation; the recovery rate is often very low.138 Therefore, the applied voltage must be optimized to ensure cell integrity during electroporation.
Hypotonic Swelling
Hypotonic hemolysis is commonly used in RBC encapsulation with better drug loading efficiency than electroporation.139 Under osmotic pressure, erythrocytes swell to form a sphere in hypotonic solutions; enlarged holes in the cell membranes allow therapeutic molecules to pass through. The RBCs then return to their regular biconcave shape and close the membrane pores in isotonic buffers.140,141 Because of the unbalanced state, osmolarity considerably influences drug loading as the environment of cells changes.142 Schnure et al demonstrated this method’s advantages by showing that β-glucosidase and β-galactosidase loaded into erythrocytes by hypotonicity-mediated hemolysis did not significantly increase cell volume. Efficient enzyme loading occurred within 60 seconds and did not affect total cell number. This new approach can potentially offer the ability to replace enzymes lost in certain disorders, such as Gaucher’s disease.19
Surface Attachment
One strategy to use cells as a drug delivery system includes immobilizing therapeutic agents on the cell surface. Surface attachment is a promising method for the enhanced stability of cell-drug interactions and can be achieved through noncovalent coupling, covalent conjugation, ligand-receptor binding, and biotinylation.
Non-Covalent Coupling
Noncovalent surface modification involves adsorbing therapeutic agents on the cell surface via intermolecular hydrogen bonds, van der Waals force, hydrophobic contact, or other forces.143 To demonstrate the application of this technique, Chambers and Mitragotri anchored polystyrene nanoparticles on the membrane of erythrocytes by noncovalent adsorption via incubation, which prevented RES clearance and prolonged circulation of 450 nm sized nanoparticles up to 15 days after adsorption onto RBCs.144 Noncovalent surface modification is relatively simple but has low stability.
Covalent Conjugation
Most cells have several functional groups on their surface, such as thiols, amines, and sialic acid residues, which can be conjugated to therapeutic agents through covalent bonds. These functional groups have the advantages of being accessible for easy labeling and low reaction toxicity. N-hydroxysuccinimide (NHS) and N-hydroxysulfosuccinimide cross-linkers are the most common reagents for primary amine modification via carbodiimide reactions.145,146 Thiol groups of cysteine-containing membrane proteins can be coupled through maleimide-thiol conjugation. Additionally, there is evidence the attachment of NPs does not affect lymphocyte activation or stem cell tumor-homing abilities.147,148 To support this claim, Stephan et al reported maleimide-functionalized liposomes covalently attached to thiol-rich T cells allowed 100 liposomes conjugated per cell without inducing toxicity or interfering with cell function such as proliferation, cytokine release, and killing of target cells.147
Covalent conjugation has a stronger binding force than adsorption and prevents therapeutic agents from detaching during cell migration. In addition, conjugation reactions to thiol or amino groups naturally expressed on the cell surface allow for exclusive covalent linkages; these covalent bonds avoid potential damage caused by encapsulation techniques and maintain the ability for controlled drug release.74 Furthermore, covalently linked drugs do not face diffusion limitations.
Ligand-Receptor Binding
Ligand–receptor interactions are fundamental for cells to communicate with one another and their environments. Cellular receptors are typically embedded within the plasma membrane and require specific ligand binding. Some receptors are exclusively found in specific cell types and can be targeted through receptor-binding moieties. Therefore, this method can be used to load particles onto cells.
Ligand-receptor binding is noncovalent, includes high specificity, avidity and offers negligible effects on cells as chemical modifications are not required. It also achieves in vivo specific binding post intravenous administration.146 Drug carriers functionalized with targeting ligands, such as antibodies, aptamers, or receptor peptides, can bind to a specific receptor expressed on the plasma membrane.149 For example, a silica nanorattle-doxorubicin loaded drug carrier functionalized with CD90 antibodies specifically anchored to MSCs via antibody-antigen binding and demonstrated efficient tumor targeting without toxicity. Further In vivo tests displayed DOX-loaded MSCs selectively accumulated in U251 glioma tumor cells and increased intratumoral distribution and retention of DOX compared to free DOX or silica nanorattle-encapsulated DOX.150 However, improving the binding efficiency to specific cell types remains challenging.
Biotinylation
Biotinylated cells can be conjugated with a wide range of proteins, enzymes, and nanoparticles without losing their biological characteristics.151 For example, the interaction between biotin and avidin is one of nature’s most powerful noncovalent interactions and is at least 10,000 times more stable than antibody-antigen interactions. Biotin is a water-soluble vitamin that has a high affinity for avidin and streptavidin tetrameric proteins; these proteins serve as biotin linkers with high specificity and affinity (equilibrium dissociation constant [Kd] 10–15 M).152 Biotin moieties can be introduced into the cell membrane by covalently coupling modified biotin molecules to the amino groups of the cell surface using, for example, NHS ester chemistry or oxidizing sialic acid hydroxyl groups to generate aldehyde moieties and then reacting them with hydrazide-modified biotin.153,154 Mooney et al attached streptavidin-conjugated polystyrene NPs on the surface of biotinylated human NSCs, improving the NPs tumor-selective distribution and retention time in vivo 4 days post injection despite the injection method (intracerebral, intravenously or contralaterally).155 Other approaches involve employing antibodies to attach biotin molecules to the cell membrane.156,157
Although nanocarrier anchoring does not affect cell function; however, the main disadvantage is using avidin or streptavidin, which are immunogenic in vivo.158 As a result, this approach to cell functionalization cannot be used in therapeutic settings. In contrast, conjugating a drug onto the cell membrane via thiol–maleimide chemistry is more practical for in vivo application; this cell modification has no effect on cell viability, proliferation, or multipotency.
Clinical Translation of Cell-Based DDS
Living cells have been used in several clinical trials for the targeted delivery of therapeutic agents. Some clinical studies of cell-based delivery are listed in Table 2.
 |
Table 2 Clinical Trials of Cell-Based Delivery Systems |
Cell-based DDS have been approved to treat various cancers. Since tumor cells such as lymphatic tumor cells cannot synthesize L-asparagine, an amino acid which promotes cell growth, L-asparaginase has been studied as anticancer agent.159 To reduce immunological adverse effects, L-asparaginase was encapsulated into RBCs by hypotonic method.160 Based on early studies that had demonstrated the effectiveness and biosafety of RBCs encapsulating L-asparaginase, several therapeutic products have been developed and conducted into clinical trials for acute lymphocytic leukemia (NCT01518517), pancreatic cancer (NCT03665441), breast cancer (NCT03674242), and so on. The first therapeutic cancer vaccine is the DC vaccine, Sipuleucel-T approved by FDA in 2010 (STN: BL 125197) for patients with metastatic hormone-refractory prostate cancer.161 The precise mechanism of action is unknown. Sipuleucel-T, an autologous cellular immunological agent, is thought to work through APCs to stimulate T-cell immune response targeted against prostatic acid phosphatase (PAP), an antigen that is highly expressed in most prostate cancer cells.162 To treat glioblastoma (GBM) in the clinic, genetically modified allogenic NSCs were used to assess the safety in 2013 (NCT01172964).163,164 Subsequently, this study provides initial clinical evidence that cytosine deaminase–expressing NSCs can migrate to the tumor site post-intracranial administration and mediate brain-localized conversion of prodrug, 5-fluorocytosine (5-FC), to the active cytotoxic agent, 5-fluorouracil (5-FU). These findings pave the way for future research into NSC-based anticancer methods for the treatment of primary and metastatic brain cancers.163
Some research has shown cell-mediated delivery can improve inflammatory diseases. Chronic pulmonary disease (COPD) is a fatal disease and its pathogenic factor is inflammation of the airways. To relieve symptoms of COPD, human RBCs from patients with COPD were loaded with increasing amounts of anti-inflammatory drug, dexamethasone 21-phosphate (Dex-21-P), and re-infused into the original donor. The drug-loaded erythrocytes acted as circulating bioreactors, converting the non-diffusible Dex-21-P into the diffusible dexamethasone.165 Especially, for inflammatory disease treatment, stem cells have widely been used due to their intrinsic therapeutic effects to relieve inflammation and promote tissue repair. MSCs can down-regulate inflammatory responses by producing anti-inflammatory cytokines and growth factors to promote tissue repair.166 For example, to treat Crohn’s disease, MSC-based drug named PROCHYMAL (NCT00482092) and Cx601 (NCT03279081) naturally migrated to defect areas and showed therapeutic outcomes, without the need of an additional drug. The safety and effectiveness of stem cells have been extensively researched and are actively being tested in clinical trials.
Most clinical trials of cell-mediated DDS have focused on cancerous or inflammatory diseases. There is a need to learn the potential impact of cell-mediated DDS in other disease types such as infectious, auto immune, and genetic diseases.
Conclusion
DDSs are extensively applicable for effectively treating cancers, inflammatory diseases, infections, and more. Cell-based delivery systems are an attractive method to overcome the limitations of conventional DDSs. Herein, we describe common cell-mediated DDSs derived from circulating cells, such as blood cells, immune cells, and stem cells. The advantages of these cells include superior biocompatibility, non-immunogenicity, long circulation times, barrier crossing capability, and natural tumor tropism, which supports that they are promising candidates for drug delivery. Although cell-based DDSs have contributed to improving diagnostic and therapeutic effects, there are still many considerations and challenges. First, therapeutic cargo must not cause toxicity to the cell carrier. To prevent this, drugs can be loaded inside vehicles, such as NPs or microparticles, liposomes, and polymers, rather than being freely encapsulated or conjugated with living cells. Second, preserving cell integrity is crucial during surface modifications. Additionally, the process of isolating cells in vivo, loading drugs, and reinjecting them back into the patient has the risk of altering the cell’s original properties. Third, cell-based DDSs face a gap between the theoretical mechanism and practical in vivo outcomes compared to traditional DDSs.
Furthermore, the mechanism and development of various diseases are still unclear. Therefore, the in vivo fate of cell-mediated DDSs must be studied further to obtain information on optimal drug delivery design, ideal administration routes, and the pathological context of cells at diseased sites. Even though there is still a wide range of considerations and challenges, with the continuous advancement of this field, we expect that cell-based DDSs will demonstrate an effective means for the precise treatment of diseases.
Acknowledgments
This research was supported by the Chung-Ang University Graduate Research Scholarship in 2021 and supported by the National Research Foundation of Korea (NRF) funded by Ministry of Science and ICT (NRF-2021R1A2C2007189, NRF-2021R1A4A3025206).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Allen TM, Culllis PR. Drug delivery systems: entering the mainstream. Science. 2004;303(5665):1818–1822.
2. Vargason AM, Anselmo AC, Mitragotri S. The evolution of commercial drug delivery technologies. Nat Biomed Eng. 2021;5(9):951–967.
3. Chi J, Ma Q, Shen Z, et al. Targeted nanocarriers based on iodinated-cyanine dyes as immunomodulators for synergistic phototherapy. Nanoscale. 2020;12(20):11008–11025.
4. Kolluru LP, Rizvi SA, D’Souza M, D’Souza MJ. Formulation development of albumin based theragnostic nanoparticles as a potential delivery system for tumor targeting. J Drug Target. 2013;21(1):77–86.
5. Hosseinidoust Z, Mostaghaci B, Yasa O, Park BW, Singh AV, Sitti M. Bioengineered and biohybrid bacteria-based systems for drug delivery. Adv Drug Deliv Rev. 2016;106(Pt A):27–44.
6. Ma Y, Nolte RJ, Cornelissen JJ. Virus-based nanocarriers for drug delivery. Adv Drug Deliv Rev. 2012;64(9):811–825.
7. Li J, Zhao J, Tan T, et al. Nanoparticle drug delivery system for glioma and its efficacy improvement strategies: a comprehensive review. Int J Nanomedicine. 2020;15:2563–2582.
8. Wu Y, Liu Y, Wang T, Jiang Q, Xu F, Liu Z. Living cell for drug delivery. Eng Regen. 2022;3(2):131–148.
9. Moon JJ, Huang B, Irvine DJ. Engineering nano- and microparticles to tune immunity. Adv Mater. 2012;24(28):3724–3746.
10. Hu CM, Fang RH, Zhang L. Erythrocyte-inspired delivery systems. Adv Healthc Mater. 2012;1(5):537–547.
11. Bhateria M, Rachumallu R, Singh R, Bhatta RS. Erythrocytes-based synthetic delivery systems: transition from conventional to novel engineering strategies. Expert Opin Drug Deliv. 2014;11(8):1219–1236.
12. Brahler M, Georgieva R, Buske N, et al. Magnetite-loaded carrier erythrocytes as contrast agents for magnetic resonance imaging. Nano Lett. 2006;6(11):2505–2509.
13. Villa CH, Anselmo AC, Mitragotri S, Muzykantov V. Red blood cells: supercarriers for drugs, biologicals, and nanoparticles and inspiration for advanced delivery systems. Adv Drug Deliv Rev. 2016;106(Pt A):88–103.
14. Fraternale A, Casabianca A, Rossi L, et al. Erythrocytes as carriers of reduced glutathione (GSH) in the treatment of retroviral infections. J Antimicrob Chemother. 2003;52(4):551–554.
15. Bossa F, Annese V, Valvano MR, et al. Erythrocytes-mediated delivery of dexamethasone 21-phosphate in steroid-dependent ulcerative colitis: a randomized, double-blind Sham-controlled study. Inflamm Bowel Dis. 2013;19(9):1872–1879.
16. Kontos S, Kourtos IC, Dane KY, Hubbell JA. Engineering antigens for in situ erythrocyte binding induces T-cell deletion. Proc Natl Acad Sci U S A. 2013;110(1):E60–E68.
17. Phua KK, Boczkowski D, Dannull J, Pruitt S, Leong KW, Nair SK. Whole blood cells loaded with messenger RNA as an anti-tumor vaccine. Adv Healthc Mater. 2014;3(6):837–842.
18. Pishesha N, Bilate AM, Wibowo MC, et al. Engineered erythrocytes covalently linked to antigenic peptides can protect against autoimmune disease. Proc Natl Acad Sci U S A. 2017;114(12):3157–3162.
19. Ihler GM, Glew RH, Schnure FW. Enzyme loading of erythrocytes. Proc Natl Acad Sci U S A. 1973;70(9):2663–2666.
20. Koleva L, Bovt E, Ataullakhanov F, Sinauridze E. Erythrocytes as carriers: from drug delivery to biosensors. Pharmaceutics. 2020;12(3):276.
21. Li Y, Raza F, Liu Y, et al. Clinical progress and advanced research of red blood cells based drug delivery system. Biomaterials. 2021;279:121202.
22. Semple JW, Italiano JE, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264–274.
23. Wang H, Wu J, Williams GR, et al. Platelet-membrane-biomimetic nanoparticles for targeted antitumor drug delivery. J Nanobiotechnology. 2019;17(1):60.
24. Buergy D, Wenz F, Groden C, Brockman MA. Tumor-platelet interaction in solid tumors. Int J Cancer. 2012;130(12):2747–2760.
25. Borsig L. The role of platelet activation in tumor metastasis. Expert Rev Anticancer Ther. 2008;8(8):1247–1255.
26. Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13(9):655–672.
27. Albarran B, Hoffman AS, Stayton PS. Efficient intracellular delivery of a pro-apoptotic peptide with A pH-responsive carrier. React Funct Polym. 2011;71(3):261–265.
28. Larochelle C, Alvarez JI, Prat A. How do immune cells overcome the blood-brain barrier in multiple sclerosis? FEBS Lett. 2011;585(23):3770–3780.
29. Varatharaj A, Galea I. The blood-brain barrier in systemic inflammation. Brain Behav Immun. 2017;60:1–12.
30. Zhang W, Wang M, Tang W, et al. Nanoparticle-laden macrophages for tumor-tropic drug delivery. Adv Mater. 2018;30(50):e1805557.
31. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–175.
32. Kitamura T, Qian BZ, Pollard JW. Immune cell promotion of metastasis. Nat Rev Immunol. 2015;15(2):73–86.
33. Smith JA. Neutrophils, host defense, and inflammation: a double-edged sword. J Leukoc Biol. 1994;56(6):672–686.
34. Chu D, Gao J, Wang Z. Neutrophil-mediated delivery of therapeutic nanoparticles across blood vessel barrier for treatment of inflammation and infection. ACS nano. 2015;9(12):11800–11811.
35. De Bondt M, Hellings N, Opdenakker G, Struyf S. Neutrophils: underestimated players in the pathogenesis of multiple sclerosis (MS). Int J Mol Sci. 2020;21(12):4558.
36. Dong Y, Lagarde J, Xicota L, et al. Neutrophil hyperactivation correlates with Alzheimer’s disease progression. Ann Neurol. 2018;83(2):387–405.
37. Price CJ, Menon DK, Peters AM, et al. Cerebral neutrophil recruitment, histology, and outcome in acute ischemic stroke: an imaging-based study. Stroke. 2004;35(7):1659–1664.
38. Jickling GC, Liu D, Ander BP, Stamova B, Zhan X, Sharp FR. Targeting neutrophils in ischemic stroke: translational insights from experimental studies. J Cereb Blood Flow Metab. 2015;35(6):888–901.
39. Szmydynger-Chodobska J, Strazielle N, Zink BJ, Ghersi-Egea JF, Chodobski A. The role of the choroid plexus in neutrophil invasion after traumatic brain injury. J Cereb Blood Flow Metab. 2009;29(9):1503–1516.
40. Clark RS, Schiding JK, Kaczorowski SL, Marion DW, Kochanek PM. Neutrophil accumulation after traumatic brain injury in rats: comparison of weight drop and controlled cortical impact models. J Neurotrauma. 1994;11(5):499–506.
41. Aizik G, Grad E, Golomb G. Monocyte-mediated drug delivery systems for the treatment of cardiovascular diseases. Drug Deliv Transl Res. 2018;8(4):868–882.
42. Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661.
43. Smith BR, Ghosn EE, Rallapalli H, et al. Selective uptake of single-walled carbon nanotubes by circulating monocytes for enhanced tumour delivery. Nat Nanotechnol. 2014;9(6):481–487.
44. Serhan CN, Ward PA, Gilroy DW. Fundamentals of Inflammation. Cambridge University Press; 2010.
45. Kapellos TS, Taylor L, Lee H, et al. A novel real time imaging platform to quantify macrophage phagocytosis. Biochem Pharmacol. 2016;116:107–119.
46. Hirayama D, Iida T, Nakase H. The phagocytic function of macrophage-enforcing innate immunity and tissue homeostasis. Int J Mol Sci. 2017;19(1):92.
47. Dong X, Chu D, Wang Z. Leukocyte-mediated delivery of nanotherapeutics in inflammatory and tumor sites. Theranostics. 2017;7(3):751–763.
48. Anand RJ, Kohler JW, Cavallo JA, Li J, Dubowski T, Hackam DJ. Toll-like receptor 4 plays a role in macrophage phagocytosis during peritoneal sepsis. J Pediatr Surg. 2007;42(6):927–932.
49. Torres FG, Troncoso OP, Pisani A, Gatto F, Bardi G. Natural polysaccharide nanomaterials: an overview of their immunological properties. Int J Mol Sci. 2019;20(20):5092.
50. Suits AG, Chait A, Aviram M, Heinecke JW. Phagocytosis of aggregated lipoprotein by macrophages: low density lipoprotein receptor-dependent foam-cell formation. Proc Natl Acad Sci U S A. 1989;86(8):2713–2717.
51. Yousefpour P, Chikoti A. Co-opting biology to deliver drugs. Biotechnol Bioeng. 2014;111(9):1699–1716.
52. Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167(3):627–635.
53. Klimp AH, De Vries EG, Scherphof GL, Daemen T. A potential role of macrophage activation in the treatment of cancer. Crit Rev Oncol Hematol. 2002;44(2):143–161.
54. Zhao Y, Haney MJ, Mahajan V, et al. Active targeted macrophage-mediated delivery of catalase to affected brain regions in models of parkinson’s disease. J Nanomed Nanotechnol. 2011;S4:003.
55. Yoo JW, Irvine DJ, Discher DE, Mitragotri S. Bio-inspired, bioengineered and biomimetic drug delivery carriers. Nat Rev Drug Discov. 2011;10(7):521–535.
56. Ma Q, Cao J, Gao Y, et al. Microfluidic-mediated nano-drug delivery systems: from fundamentals to fabrication for advanced therapeutic applications. Nanoscale. 2020;12(29):15512–15527.
57. Jones RB, Mueller S, Kumari S, et al. Antigen recognition-triggered drug delivery mediated by nanocapsule-functionalized cytotoxic T-cells. Biomaterials. 2017;117:44–53.
58. Lanzavecchia A, Iezzi G, Viola A. From TCR engagement to T cell activation: a kinetic view of T cell behavior. Cell. 1999;96(1):1–4.
59. Singh N, Lee YG, Shestova O, et al. Impaired death receptor signaling in leukemia causes antigen-independent resistance by inducing CAR T-cell dysfunction. Cancer Discov. 2020;10(4):552–567.
60. Ivica NA, Young CM. Tracking the CAR-T revolution: analysis of clinical trials of CAR-T and TCR-T therapies for the treatment of cancer (1997–2020). Healthcare. 2021;9(8):1062.
61. Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11(4):69.
62. Siriwon N, Kim YJ, Siegler E, et al. CAR-T cells surface-engineered with drug-encapsulated nanoparticles can ameliorate intratumoral T-cell hypofunction. Cancer Immunol Res. 2018;6(7):812–824.
63. Jin C, Fotaki G, Ramachandran M, Nilsson B, Essand M, Yu D. Safe engineering of CAR T cells for adoptive cell therapy of cancer using long-term episomal gene transfer. EMBO Mol Med. 2016;8(7):702–711.
64. Park TS, Rosenberg SA, Morgan RA. Treating cancer with genetically engineered T cells. Trends Biotechnol. 2011;29(11):550–557.
65. Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 2020;21(8):835–847.
66. Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat Rev Clin Oncol. 2021;18(2):85–100.
67. Smyth MJ, Cretney E, Kelly JM, et al. Activation of NK cell cytotoxicity. Mol Immunol. 2005;42(4):501–510.
68. Wang W, Erbe AK, Hank JA, Morris ZS, Sondel PM. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front Immunol. 2015;6:368.
69. Martinet L, Smyth MJ. Balancing natural killer cell activation through paired receptors. Nat Rev Immunol. 2015;15(4):243–254.
70. Deng G, Sun Z, Li S, et al. Cell-membrane immunotherapy based on natural killer cell membrane coated nanoparticles for the effective inhibition of primary and abscopal tumor growth. ACS Nano. 2018;12(12):12096–12108.
71. Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L. Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol. 2014;44(6):1582–1592.
72. Zhang C, Burger MC, Jennewein L, et al. ErbB2/HER2-specific NK cells for targeted therapy of glioblastoma. J Natl Cancer Inst. 2015;108:5.
73. Valipour B, Velaei K, Abedelahi A, Karimipour M, Darabi M, Charoudeh HN. NK cells: an attractive candidate for cancer therapy. J Cell Physiol. 2019;234(11):19352–19365.
74. Su Y, Xie Z, Kim GB, Dong C, Yang J. Design strategies and applications of circulating cell-mediated drug delivery systems. ACS Biomater Sci Eng. 2015;1(4):201–217.
75. Teng CF, Jeng LB, Shyu WC. Role of insulin-like growth factor 1 receptor signaling in stem cell stemness and therapeutic efficacy. Cell Transplant. 2018;27(9):1313–1319.
76. Mills KM, Szczerkowski JLA, Habib SJ. Wnt ligand presentation and reception: from the stem cell niche to tissue engineering. Open Biol. 2017;7(8):170140.
77. Han Y, Li X, Zhang Y, Han Y, Chang F, Ding J. Mesenchymal stem cells for regenerative medicine. Cells. 2019;8(8):886.
78. Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration. 2013;85(1):3–10.
79. Tang J, Wang J, Huang K, et al. Cardiac cell-integrated microneedle patch for treating myocardial infarction. Sci Adv. 2018;4(11):eaat9365.
80. Salehi H, Al-Arag S, Middendorp E, Gergely C, Cuisinier F, Orti V. Dental pulp stem cells used to deliver the anticancer drug paclitaxel. Stem Cell Res Ther. 2018;9(1):103.
81. Fu H, Wu Y, Yang X, et al. Stem cell and its derivatives as drug delivery vehicles: an effective new strategy of drug delivery system. AllLife. 2021;14(1):782–798.
82. Xie C, Yang Z, Suo Y, et al. Systemically infused mesenchymal stem cells show different homing profiles in healthy and tumor mouse models. Stem Cells Transl Med. 2017;6(4):1120–1131.
83. Kean TJ, Lin P, Caplan AI, Dennis JE. MSCs: delivery routes and engraftment, cell-targeting strategies, and immune modulation. Stem Cells Int. 2013;2013:732742.
84. Xia J, Tsai AC, Cheng W, Yuan X, Ma T, Guan J. Development of a microdevice-based human mesenchymal stem cell-mediated drug delivery system. Biomater Sci. 2019;7(6):2348–2357.
85. Wang X, Chen H, Zeng X, et al. Efficient lung cancer-targeted drug delivery via a nanoparticle/MSC system. Acta Pharm Sin B. 2019;9(1):167–176.
86. Wang X, Gao J, Ouyang X, Wang J, Sun X, Lv Y. Mesenchymal stem cells loaded with paclitaxel-poly (lactic- co-glycolic acid) nanoparticles for glioma-targeting therapy. Int J Nanomedicine. 2018;13:5231–5248.
87. Hu Q, Sun W, Wang J, et al. Conjugation of haematopoietic stem cells and platelets decorated with anti-PD-1 antibodies augments anti-leukaemia efficacy. Nat Biomed Eng. 2018;2(11):831–840.
88. Su Y, Zhang T, Huang T, Gao J. Current advances and challenges of mesenchymal stem cells-based drug delivery system and their improvements. Int J Pharm. 2021;600:120477.
89. Idorn M, Skadborg SK, Kellermann L, et al. Chemokine receptor engineering of T cells with CXCR2 improves homing towards subcutaneous human melanomas in xenograft mouse model. Oncoimmunology. 2018;7(8):e1450715.
90. D’souza N, Burns JS, Grisendi G, et al. MSC and tumors: homing, differentiation, and secretion influence therapeutic potential. Adv Biochem Eng Biotechnol. 2013;130:209–266.
91. Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84(7):2068–2101.
92. Krueger TEG, Thorek DLJ, Denmeade SR, Isaacs JT, Brennen WN. Concise review: mesenchymal stem cell-based drug delivery: the good, the bad, the ugly, and the promise. Stem Cells Transl Med. 2018;7(9):651–663.
93. Tiet P, Berlin JM. Exploiting homing abilities of cell carriers: targeted delivery of nanoparticles for cancer therapy. Biochem Pharmacol. 2017;145:18–26.
94. Summers C, Rankin SM, Condliffe AM, Singh N, Peters AM, Chilvers ER. Neutrophil kinetics in health and disease. Trends Immunol. 2010;31(8):318–324.
95. Strieter RM, Lukacs NW, Standiford TJ, Kunkel SL. Cytokines. 2. Cytokines and lung inflammation: mechanisms of neutrophil recruitment to the lung. Thorax. 1993;48(7):765–769.
96. Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nat Med. 2011;17(11):1381–1390.
97. Zarbock A, Ley K, McEver RP, Hidalgo A. Leukocyte ligands for endothelial selectins: specialized glycoconjugates that mediate rolling and signaling under flow. Blood. 2011;118(26):6743–6751.
98. Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7(9):678–689.
99. Massena S, Christoffersson G, Hjertstrom E, et al. A chemotactic gradient sequestered on endothelial heparan sulfate induces directional intraluminal crawling of neutrophils. Blood. 2010;116(11):1924–1931.
100. BcDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362–366.
101. Luster AD, Alon R, Andrian UH. Immune cell migration in inflammation: present and future therapeutic targets. Nat Immunol. 2005;6(12):1182–1190.
102. Sackstein R, Schatton T, Barthel SR. T-lymphocyte homing: an underappreciated yet critical hurdle for successful cancer immunotherapy. Lab Invest. 2017;97(6):669–697.
103. Becker AD, Riet IV. Homing and migration of mesenchymal stromal cells: how to improve the efficacy of cell therapy? World J Stem Cells. 2016;8(3):73–87.
104. None TM, None AC, SH Ranganath. Bioengineered cellular and cell membrane-derived vehicles for actively targeted drug delivery: so near and yet so far. Adv Drug Deliv Rev. 2018;132:57–80.
105. Roger M, Clavreul A, Venier-Julienne MC, et al. Mesenchymal stem cells as cellular vehicles for delivery of nanoparticles to brain tumors. Biomaterials. 2010;31(32):8393–8401.
106. Aleynik A, Gernavage KM, Mourad YS, et al. Stem cell delivery of therapies for brain disorders. Clin Transl Med. 2014;3:24.
107. Lopez-Santalla M, Hervas-Salcedo R, Fernandez-Garcia M, Bueren JA, Garin MI. Cell therapy with mesenchymal stem cells induces an innate immune memory response that attenuates experimental colitis in the long term. J Crohns Colitis. 2020;14(10):1424–1435.
108. Porada CD, Almeida-Porada G. Mesenchymal stem cells as therapeutics and vehicles for gene and drug delivery. Adv Drug Deliv Rev. 2010;62(12):1156–1166.
109. Höckel M, Vaupel P. Tumor hypoxia: definitions and current clinical, biologic, and molecular aspects. J Natl Cancer Inst. 2001;93(4):266–276.
110. Pattabiraman DR, Weinberg RA. Tackling the cancer stem cells – what challenges do they pose? Nat Rev Drug Discov. 2014;13(7):497–512.
111. Winner M, Koong AC, Rendon BE, Zundel W, Mitchell RA. Amplification of tumor hypoxic responses by macrophage migration inhibitory factor-dependent hypoxia-inducible factor stabilization. Cancer Res. 2007;67(1):186–193.
112. Konisti S, Kiriakidis S, Paleolog EM. Hypoxia–A key regulator of angiogenesis and inflammation in rheumatoid arthritis. Nat Rev Rheumatol. 2012;8(3):153–162.
113. Finger EC, Giaccia AJ. Hypoxia, inflammation, and the tumor microenvironment in metastatic disease. Cancer Metastasis Rev. 2010;29(2):285–293.
114. Gregory JL, Morand EF, McKeown SJ, et al. Macrophage migration inhibitory factor induces macrophage recruitment via CC chemokine ligand 2. J Immunol. 2006;177(11):8072–8079.
115. Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. 2011;11(9):597–606.
116. Goswami KK, Ghosh T, Ghosh S, Sarkar M, Bose A, Baral R. Tumor promoting role of anti-tumor macrophages in tumor microenvironment. Cell Immunol. 2017;316:1–10.
117. Yang L, Zhang Y. Tumor-associated macrophages: from basic research to clinical application. J Hematol Oncol. 2017;10(1):58.
118. Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41(1):49–61.
119. Spaeth E, Klopp A, Dembinski J, Andreeff M, Marini F. Inflammation and tumor microenvironments: defining the migratory itinerary of mesenchymal stem cells. Gene Ther. 2008;15(10):730–738.
120. Liesveld JL, Sharma N, Aljitawi OS. Stem cell homing: from physiology to therapeutics. Stem Cells. 2020;38(10):1241–1253.
121. Domanska UM, Kruizinga RC, Nagengast WB, et al. A review on CXCR4/CXCL12 axis in oncology: no place to hide. Eur J Cancer. 2013;49(1):219–230.
122. Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiol Rev. 1997;77(3):759–803.
123. Doherty GJ, McMahon HT. Mechanisms of endocytosis. Annu Rev Biochem. 2009;78:857–902.
124. Gordon S. Phagocytosis: an immunobiologic process. Immunity. 2016;44(3):463–475.
125. Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17(1):593–623.
126. Batrakova EV, Gendelman HE, Kabanov AV. Cell-mediated drug delivery. Expert Opin Drug Deliv. 2011;8(4):415–433.
127. Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66(17):2873–2896.
128. Lin SY, Hsu WH, Lo JM, Tsai HC, Hsiue GH. Novel geometry type of nanocarriers mitigated the phagocytosis for drug delivery. J Control Release. 2011;154(1):84–92.
129. Champion JA, Katare YK, Mitragotri S. Making polymeric micro- and nanoparticles of complex shapes. Proc Natl Acad Sci U S A. 2007;104(29):11901–11904.
130. Champion JA, Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103(13):4930–4934.
131. Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol Scand. 2003;177(4):437–447.
132. Xie Z, Su Y, Kim GB, et al. Immune cell-mediated biodegradable theranostic nanoparticles for melanoma targeting and drug delivery. Small. 2017;13(10):10.
133. Lizano C, Sanz S, Luque J, Pinilla M. In vitro study of alcohol dehydrogenase and acetaldehyde dehydrogenase encapsulated into human erythrocytes by an electroporation procedure. Biochim Biophys Acta. 1998;1425(2):328–336.
134. Kim SH, Kim EJ, Hou JH, et al. Opsonized erythrocyte ghosts for liver-targeted delivery of antisense oligodeoxynucleotides. Biomaterials. 2009;30(5):959–967.
135. Chen FG, Wang C, Zhi DY, Xia GM. Analysis of amino acids in individual wheat embryonic protoplast. Amino Acids. 2005;29(3):235–239.
136. Ponsaerts P, Bosch GV, Cools N, et al. Messenger RNA electroporation of human monocytes, followed by rapid in vitro differentiation, leads to highly stimulatory antigen-loaded mature dendritic cells. J Immunol. 2002;169(4):1669–1675.
137. Prechtel AT, Turza NM, Theodoridis AA, Kummer M, Steinkasserer A. Small interfering RNA (siRNA) delivery into monocyte-derived dendritic cells by electroporation. J Immunol Methods. 2006;311(1–2):139–152.
138. He H, Ye J, Wang Y, et al. Cell-penetrating peptides meditated encapsulation of protein therapeutics into intact red blood cells and its application. J Control Release. 2014;176:123–132.
139. López SC, Meissner KE. Characterization of carrier erythrocytes for biosensing applications. J Biomed Opt. 2017;22(9):91510.
140. Sanz S, Lizano C, Luque J, Pinilla M. In vitro and in vivo study of glutamate dehydrogenase encapsulated into mouse erythrocytes by a hypotonic dialysis procedure. Life Sci. 1999;65(26):2781–2789.
141. Hamidi M, Tajerzadeh H. Carrier erythrocytes: an overview. Drug Deliv. 2003;10(1):9–20.
142. Markov DE, Boeve H, Gleich B, et al. Human erythrocytes as nanoparticle carriers for magnetic particle imaging. Phys Med Biol. 2010;55(21):6461–6473.
143. Gao M, Hu A, Sun X, et al. Photosensitizer decorated red blood cells as an ultrasensitive light-responsive drug delivery system. ACS Appl Mater Interfaces. 2017;9(7):5855–5863.
144. Chambers E, Mitragotri S. Prolonged circulation of large polymeric nanoparticles by non-covalent adsorption on erythrocytes. J Control Release. 2004;100(1):111–119.
145. Lee DY, Cha BH, Jung M, Kim AS, Bull DA, Won YW. Cell surface engineering and application in cell delivery to heart diseases. J Biol Eng. 2018;12:28.
146. Yu H, Yang Z, Li F, Xu L, Sun Y. Cell-mediated targeting drugs delivery systems. Drug Deliv. 2020;27(1):1425–1437.
147. Stephan MT, Moon JJ, Um SH, Bershteyn A, Irvine DJ. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nat Med. 2010;16(9):1035–1041.
148. Kim H, Shin K, Park OK, et al. General and facile coating of single cells via mild reduction. J Am Chem Soc. 2018;140(4):1199–1202.
149. Swiston AJ, Cheng C, Um SH, Irvine DJ, Cohen RE, Rubner MF. Surface functionalization of living cells with multilayer patches. Nano Lett. 2008;8(12):4446–4453.
150. Li L, Guan Y, Liu H, et al. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano. 2011;5(9):7462–7470.
151. Parrott MB, Adams KE, Mercier GT, Mok H, Campos SK, Barry MA. Metabolically biotinylated adenovirus for cell targeting, ligand screening, and vector purification. Mol Ther. 2003;8(4):688–700.
152. Thomsen T, Klok HA. Chemical cell surface modification and analysis of nanoparticle-modified living cells. ACS Appl Bio Mater. 2021;4(3):2293–2306.
153. Cheng H, Kastrup CJ, Ramanathan R, et al. Nanoparticulate cellular patches for cell-mediated tumoritropic delivery. ACS Nano. 2010;4(2):625–631.
154. Yao S, Li X, Liu J, Sun Y, Wang Z, Jiang Y. Maximized nanodrug-loaded mesenchymal stem cells by a dual drug-loaded mode for the systemic treatment of metastatic lung cancer. Drug Deliv. 2017;24(1):1372–1383.
155. Mooney R, Weng Y, Tirughana-Sambandan R, et al. Neural stem cells improve intracranial nanoparticle retention and tumor-selective distribution. Future Oncol. 2014;10(3):401–415.
156. Sakahara H, Saga T. Avidin-biotin system for delivery of diagnostic agents. Adv Drug Deliv Rev. 1999;37(1–3):89–101.
157. Ahmed KK, Geary SM, Salem AK. Surface engineering tumor cells with adjuvant-loaded particles for use as cancer vaccines. J Control Release. 2017;248:1–9.
158. Chinol M, Caslini P, Maggiolo M, et al. Biochemical modifications of avidin improve pharmacokinetics and biodistribution, and reduce immunogenicity. Br J Cancer. 1998;78(2):189–197.
159. Ravasan S, Madadi E, Fathi Z, et al. The effect of Yarrowia lipolytical-asparaginase on apoptosis induction and inhibition of growth in Burkitt’s lymphoma Raji and acute lymphoblastic leukemia MOLT-4 cells. Int J Biol Macromol. 2020;146:193–201.
160. Godfrin Y, Horand F, Franco R, et al. International seminar on the red blood cells as vehicles for drugs. Expert Opin Biol Ther. 2012;12(1):127–133.
161. Galluzzi L, Senovilla L, Vacchelli E, et al. Trial watch: dendritic cell-based interventions for cancer therapy. Oncoimmunology. 2012;1(7):1111–1134.
162. Anassi E, Ndefo UA. Sipuleucel-T (provenge) injection: the first immunotherapy agent (vaccine) for hormone-refractory prostate cancer. P T. 2011;36(4):197–202.
163. Portnow J, Synold TW, Badie B, et al. Neural stem cell-based anticancer gene therapy: a first-in-human study in recurrent high-grade glioma patients. Clin Cancer Res. 2017;23(12):2951–2960.
164. Portnow J, Badie B, Synold TW, et al. A first-in-human study of neural stem cells (NSCs) expressing cytosine deaminase (CD) in combination with 5-fluorocytosine (5-FC) in patients with recurrent high-grade glioma. J Clin Oncol. 2013;31(15):2018.
165. Rossi L, Serafini S, Cenerini L, et al. Erythrocyte-mediated delivery of dexamethasone in patients with chronic obstructive pulmonary disease. Biotechnol Appl Biochem. 2001;33(2):85–89.
166. Kurtzberg J, Prockop S, Teira P, et al. Allogeneic human mesenchymal stem cell therapy (remestemcel-L, Prochymal) as a rescue agent for severe refractory acute graft-versus-host disease in pediatric patients. Biol Blood Marrow Transplant. 2014;20(2):229–235.
167. Beduneau A, Ma Z, Grotepas CB, et al. Facilitated monocyte-macrophage uptake and tissue distribution of superparmagnetic iron-oxide nanoparticles. PLoS One. 2009;4(2):e4343.
168. Sarkar S, Thapa R, Naushin F, et al. Antibiotic-loaded smart platelet: a highly effective invisible mode of killing both antibiotic-sensitive and -resistant bacteria. ACS Omega. 2022;7(28):24102–24110.
169. Choi J, Kim HY, Ju EJ, et al. Use of macrophages to deliver therapeutic and imaging contrast agents to tumors. Biomaterials. 2012;33(16):4195–4203.
170. Lizano C, Pérez MT, Pinilla M. Mouse erythrocytes as carriers for coencapsulated alcohol and aldehyde dehydrogenase obtained by electroporation in vivo survival rate in circulation, organ distribution and ethanol degradation. Life Sci. 2001;68(17):2001–2016.
171. Levene M, Bain MD, Moran NF, et al. Safety and efficacy of erythrocyte encapsulated thymidine phosphorylase in mitochondrial neurogastrointestinal encephalomyopathy. J Clin Med. 2019;8(4):457.
172. Anselmo AC, Gupta V, Zern BJ, et al. Delivering nanoparticles to lungs while avoiding liver and spleen through adsorption on red blood cells. ACS Nano. 2013;7(12):11129–11137.
173. Stephan MT, Stephan SB, Bak P, Chen J, Irvine DJ. Synapse-directed delivery of immunomodulators using T-cell-conjugated nanoparticles. Biomaterials. 2012;33(23):5776–5787.
174. Rossi NA, Constantinescu I, Kainthan RK, Brooks DE, Scott MD, Kizhakkedathu JN. Red blood cell membrane grafting of multi-functional hyperbranched polyglycerols. Biomaterials. 2010;31(14):4167–4178.
175. Yan H, Mi X, Midgley AC, et al. Targeted repair of vascular injury by adipose-derived stem cells modified with P-selectin binding peptide. Adv Sci. 2020;7(11):1903516.
176. Xu P, Zuo H, Zhou R, et al. Doxorubicin-loaded platelets conjugated with anti-CD22 mAbs: a novel targeted delivery system for lymphoma treatment with cardiopulmonary avoidance. Oncotarget. 2017;8(35):58322–58337.
177. Xu M, Asghar S, Dai S, et al. Mesenchymal stem cells-curcumin loaded chitosan nanoparticles hybrid vectors for tumor-tropic therapy. Int J Biol Macromol. 2019;134:1002–1012.
178. Ayer M, Burri O, Guiet R, et al. Biotin-neutravidin mediated immobilization of polymer micro- and nanoparticles on T lymphocytes. Bioconjug Chem. 2021;32(3):541–552.
179. Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled, randomized trial of mesenchymal stem cells in COPD. Chest. 2013;143(6):1590–1598.




