Diagnosis of Toxoplasmosis Using Surface Antigen Grade 1 Detection by ELISA, Nano-Gold ELISA, and PCR in Pregnant Women
Introduction
Toxoplasma gondii (T. gondii) is a worldwide obligate intracellular parasite.1 According to estimates, toxoplasmosis has infected one-third of the world’s population.2,3 T. gondii IgG antibody prevalence in pregnant women was surveyed in several governorates of Egypt, ranging between 44.0 to 67.5% and 23.0% of aborted women.4 Also, the prevalence among dogs (23.3%) and cats (9.8%) in Egypt was reported.5 About 0.8% of pregnant females were confirmed to be at hazard of acute seroconversion. Pregnant women with toxoplasmosis run the risk of miscarriage, stunted growth, early delivery, or congenital anomalies (encephalitis, hydrocephalus, microcephalus, retinochoroiditis, blindness, mental retardation epilepsy).4,6 The severity of toxoplasmosis relies on the host’s immune response and the T. gondii strain.7
Toxoplasmosis can be diagnosed by various methods, including antibody detection using serological tests, and parasite DNA determination by polymerase chain reaction (PCR).
The most widely utilized technique nowadays is antibody detection, which provides serological proof of immunoglobulin (Ig)G and IgM antibodies specific to T. gondii antigens. These tests could have sensitivity and specificity problems, which could produce false-positive or false-negative results.8 The emergence of molecular biology and serological techniques coincided with the emergence of many T. gondii stage-specific genes. One of these, surface antigen grade 1 (SAG1), which is specific to tachyzoites, makes up 3.0–5.0% of the surface membrane proteins. It is essential for parasite adhesion and host-cell penetration. It also has an important role in immune modulation.9 First trials of PCR detection based on SAG1-specific primers showed that they were able to amplify DNA from seven test T. gondii strains with no cross-reactivity with other parasites or microbes. Hence, these primers are highly specific.10 The sensitivity of PCR is not only affected by the genomic copy number of the target gene but is also affected by the infection stage. PCR detection using primers specific to several target genes including SAG 1 is often very effective.11
Nano diagnostics is the application of nanotechnology in clinical diagnosis to solve the need for increased sensitivity, specificity, and early detection. The nanomaterial helps many target molecules bind more easily for ultrasensitive detection due to their large surface area.12 Different materials were used for nanoparticle synthesis and used in medical research to cure and diagnose various illnesses, such as gold, chitosan, and silver. Nowadays, using nano-gold particles as a weapon against various parasites has become a common practice in research and medicine.13 The currently available and frequently used toxoplasmosis diagnostic procedures can be improved by nanomaterials or perhaps replaced by them.14 We used pregnant women sera in our study due to the availability of routine Toxoplasma gondii screening tests during prenatal care. The aim of our study was to compare a new nano-gold ELISA with ELISA and PCR for diagnosis of toxoplasmosis using SAG1.
Patients and Methods
Sample Collection
Samples of blood were collected from pregnant women seeking antenatal care in the outpatient clinic of the Gynecology and Obstetrics Department of Benha University Hospital, Benha Teaching Hospital, and also from different laboratories using commercial IgG and IgM ELISA kits (Sigma, St. Louis, MO, USA). In the period between October 2017 and December 2020, the study protocol complies with the Declaration of Helsinki15 and was revised and approved by the Medical Research Ethical Committee at Benha Faculty of Medicine, Benha University (Approval no. MD-5-11-2017). The objective of the study was explained to all pregnant females participating in this work and informed consent was taken. The study was conducted on 87 pregnant females, and they were grouped as follows: Group I: Thirty-seven T. gondii infected patients (presence of IgM, seroconversion, or rising IgG antibodies titer in their serum). Group II: Thirty patients having other parasites than T. gondii: ten patients infected with Cryptosporidium parvum (C. parvum), 10 patients infected with Giardia lamblia (G. lamblia), and 10 patients infected with Entamoeba histolytica (E. histolytica). The idea behind including patients harboring other parasites than T. gondii in our study was to avoid cross-reactivity in the results. Group III: Twenty apparently healthy pregnant women free from parasitic infections served as noninfected control (negative control). Blood samples were stored at –20°C till required for the study tests.
Animals
Three Swiss Albino male mice, weighting roughly 20–30 grams, were used in our study in the preparation of Toxoplasma surface antigen grade I (SAG1). Mice were obtained from the animal house of Theodore Bilharz Research Institute (TBRI). They were housed in plastic cages with white wood chips for bedding, fed by commercial complete food mixture and tap water for drinking and maintained temperature (22±2). Also, two New Zealand white male rabbits, weighing approximately 1.5 Kg and about two months age, were used in the production of polyclonal antibodies (pAb). They were purchased from the Rabbit Research Unit (RRU), Agriculture Faculty, Cairo University. The rabbits were housed for four weeks (experiment duration) in the animal house in TBRI under standard laboratory care at 21°C, 16% moisture, filtered drinking water with additional salts 1cm/5 liters and vitamin 1cm/10 liters. A diet containing 15% protein, 3% fat, and 22% fiber was purchased from RRU. All procedures related to experimental animals in the present study approved by TBRI and met the International Guiding Principles for Biomedical Research Involving Animals, as issued by the International Organizations of Medical Sciences (NRC, 2006).16
ELISA Techniques
Preparation of SAG 1 Antigen
T. gondii tachyzoites (RH strain), kindly provided by Parasitology Department of (TBRI), were used for the preparation of Toxoplasma surface antigen grade I (SAG1).17 Briefly, T. gondii RH strain tachyzoites were grown and maintained in three Swiss Albino male mice by intraperitoneal inoculation. The peritoneal fluids from infected mice were pooled, centrifuged, and then cleaned three times with phosphate buffer saline every four days after infection, disrupted by sonication then centrifuged at 12,000 rpm for 1 hour, and the supernatant collected and the purified target antigen was obtained by DEAE-Sephadex A-50 ion exchange chromatography as a soluble antigen.18,19 The concentration of protein was determined dependent on Coomassie brilliant blue G-250 dye color change and the soluble antigen stored at −20°C till use. Assessment of reactivity and specificity of Toxoplasma surface antigen grade I (SAG1) was done by indirect ELISA.20
Production of Polyclonal Antibodies (pAb)
By immunizing one of the New Zealand white rabbits withSAG1, through intramuscular injection (i.m.) of a priming dose at two sites, 1mg Toxoplasma SAG1 mixed 1:1 in complete Freund’s adjuvant (CFA) (Sigma), this was followed by 3 booster doses, each was 0.5 mg antigen emulsified in equal vol. of incomplete Freund’s adjuvant (IFA) (Sigma). Two weeks after the priming dose, the first boosting was given. The following boosting doses were given at weekly intervals.21 Purification of the rabbit’s anti-Toxoplasma serum by ammonium sulfate (50%) precipitation method was done as 50% Saturated ammonium sulfate solution was added dropwise to rabbit anti-Toxoplasma serum to reach 50% saturation, with continuous stirring and then centrifuged for 20 min at 3500 rpm in a cooling centrifuge (Heraeus) at 4°C. The supernatant was discarded, and the ammonium sulfate precipitation steps were repeated several times. The final precipitate was dissolved in a suitable amount of 0.01 M PBS, pH 7.2. The ammonium sulfate was removed by dialysis against 0.01 M PBS, pH 7.2 for 72 hrs at 4°C. Then, purification by caprylic acid (7%) method was done, caprylic acid was added dropwise on the precipitated whole Igs after 50% ammonium sulfate precipitation, with slow magnetic stirring for 30 min at 4°C then the mixture was centrifugated at 1000 rpm for 30 minutes. Precipitation was discarded, while the supernatant contained nearly pure IgG.22–24
One fraction of polyclonal antibodies was conjugated with Horseradish peroxidase (HRP). This was done on two successive days. On the 1st day, (A) Five mg of horseradish peroxidase (HRP) (Sigma) was suspended in 1.2 mL of dist. H2O, then 0.3 mL (300ul) of freshly prepared sodium periodate was added and incubated at room temperature for 20 min. The HRP solution was dialyzed against 1 mM sodium acetate buffer. (B) Five mg/mL of polyclonal antibody was dialyzed against 0.02M carbonate buffer. On the 2nd day, A and B were mixed and then incubated for two hours at room temperature.100 μL of freshly prepared Na borohydride was added to the mixture of A +B and then incubated for two hours at 4 °C.25 The second fraction of polyclonal antibodies remained without conjugation. The third fraction was conjugated with nanogold particles (AuNPs-MUA) and is mentioned in the next section. The three fractions were stored at −20 °C till required.
Nano-Gold Particles (AuNPs)
AuNPs were purchased from Nanotech Company (6th October City, Egypt) and scanned by transmission electron microscope. AuNPs functionalized with mercaptoundecanoic acid (AuNPs-MUA) by adding 30 mL of the prepared AuNPs to 45 µL of 1 mMmercaptoundecanoic acid (MUA) in ethanol solution (Sigma Aldrich, Germany). The solution was mixed and left overnight at 4°C. The concentration was determined the next day by UV/vis spectrophotometry using Beer’s law.26
The 3rd fraction of polyclonal antibodies was conjugated with nanogold particles (AuNPs-MUA). Two mg of anti-Toxoplasma pAb was added to 5 mL of AuNPs-MUA (2 nM; pH = 7.4). To obtain more robust AuNPs-pAb conjugates, the covalent linkage between pAb and AuNPs-MUA was done through the use of N-hydroxy succinimide/1-Ethyl-3-[3-dimethylaminopropyl] carbodiimide (NHS/EDC) cross-linkers (NHS: Fluka, Germany). Five mL of a mixture of 5 mM sodium phosphate buffer (pH = 7), 1.2 mM NHS, and 2.8 mM EDC was added to 5 mL of AuNPs-pAb conjugates. The mixture was mixed and left to incubate overnight at 4°C.27,28
ELISA Procedure
Microtiter plates were coated with 100 µL/well of purified pAb in a concentration of 20 µg/mL as a capture antibody diluted in 0.8 M carbonate buffer, pH 9.6 (1/20). The plates were washed with a washing buffer [0.1 M PBS/0.05% Tween 20, PH 7.4 (PBS/T)] three times. The free sites were blocked with 200 µL/well of 0.1% BSA/PBS/T and incubated for 1 h at 37ᵒC. The plates were washed with 0.1 M PBS/T five times. Pregnant women’s sera (100 µL/well), in dilution 1/50, were added and incubated for 2 h at 37ᵒC, and the plates were washed 3 times. Peroxidase-conjugate pAbs (100 µL/well) in concentration 20 µg/mL diluted in PBS (1/80) were distributed, and plates were incubated for 2 h at 37ᵒC. The reactions were visualized by the addition of 100 µL/well of O-phenylenediamine substrate solution for 30 min at room temperature. The reaction was stopped by adding 50 µL/well of 8 N H2SO4, and the plates were read at optical density (OD) 490 nm with an ELISA microplate reader (Bio-Rad). The cutoff point was 0.16 in ELISA.29,30
Nano-Gold ELISA
Microtiter plates were coated with 100 µL/well of nano-gold conjugated pAb in concentration of 10 µg/mL as a capture antibody diluted in 0.8 M carbonate buffer, pH 9.6 (1/40). The plates were washed with a washing buffer [0.1 M PBS/0.05% Tween 20, PH 7.4 (PBS/T)] three times. The free sites were blocked with 200 µL/well of 0.1% BSA/PBS/T and incubated for 1 h at 37ᵒC. The plates were washed with 0.1 M PBS/T five times. Pregnant women’s sera (100 µL/well), in dilution 1/50, were added and incubated for 2 h at 37ᵒC, and the plates were washed 3 times. Peroxidase-conjugate pAbs (100 µL/well) in concentration 15µg/mL diluted in PBS (1/80) were distributed, and plates were incubated for 2 h at 37ᵒC. The reactions were visualized by the addition of 100 µL/well of O-phenylenediamine substrate solution for 30 min at room temperature. The reaction was stopped by adding 50 µL/well of 8 N H2SO4, and the plates were read at optical density (OD) 490 nm with an ELISA microplate reader (Bio-Rad). The cutoff point was 0.203 in nano-gold ELISA.29,30
Polymerase Chain Reaction (PCR)
Human DNA was extracted from blood samples by using QIAmpDNA Blood Mini Kit supplied by (Qiagen, Germany) according to the manufacturer’s instructions. After extraction, DNA samples were measured using NanoDrop 1000 Spectrophotometer (Thermo Scientific, USA). The eluted DNA was then preserved at −20°C until further processing. T. gondii major surface antigen (SAG1) gene (Accession NO. MK250980.1) was amplified using two oligonucleotide primers: F: 5´-GCTGTAACATTGAGCTCCTTGATTCCTG-3´ and R: 5´-CCGGAACAGTACTGATTGTTGTCTTGAG-3´ and Maxima Hot Start Green Master Mix (Fermentas, Thermo Fisher Scientific Co., USA). PCR reaction was conducted in a total volume of 50 µL using 25 µL of Taq PCR Master Mix, 2.5 µL forward and reverse primers,10 µL of template DNA, 10 µL of nuclease-free. An initial denaturation step at 94 °C for 5 min, then 35 cycles consisting of 94 °C for 30 sec, 62 °C for 30 sec, 72 °C for 30 sec, and a final extension step at 72 °C for 10 min. The amplified DNA of the SAG1 gene was electrophoresed with 1% agarose gel.31–33
Statistical Analysis
The collected data were analyzed by SPSS program (SpssInc, Chicago, ILL Company) version 16 software. Chi-square (χ2) and Fisher’s exact tests were used. Receiver operator characteristic (ROC) curves were constructed to assess the performance of PCR and ELISA in the diagnosis of toxoplasmosis. MedCalc Software (Mariakerke, Belgium) was used to compare areas under the curve of the 3 parameters.34,35 The data were considered significant if p-values were equal to or less than 0.05.
Results
Toxoplasma SAG1 Antigens
The SAG1 antigen was purified using DEAE Sephadex A-50 ion exchange chromatography, and the OD280 profile is obtained and shown in Figure 1. A single peak with a maximum OD value of 1.2 at fraction number 10 represents the eluted antigen (represented by the fraction with the highest protein content). We collected fraction no. 10 as SAG 1 antigen.
Anti-Toxoplasma pAb
Figure 2 shows increasing antibody level in the immunized rabbit started one week after the first booster dose. With the 3rd booster dose, the immune sera gave a high titer against Toxoplasma antigen with an OD of 1.8 at 1/250 dilution. The non-immunized rabbit showed no increase in antibody level. Table 1 shows that the produced anti-Toxoplasma antibodies diluted 1/250 in PBS/T gave strong reactivity to Toxoplasma antigen. The OD reading at 490 nm for T. gondii was 1.82 compared to 0.28, 0.2, and 0.22 for C. parvum, E. histolytica, and G. lamblia, respectively.
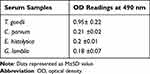 |
Table 1 Reactivity of Purified Target Antigen by ELISA |
Nano-Gold Particles (AuNPs)
Transmission electron microscopy (TEM) was used to get direct information about the size of the produced nano-gold particles. TEM images showed that most particles (66%) were approximately about 40 nm (Figure 3).
ELISA
As regards detecting Toxoplasma (SAG1) by ELISA in the serum, the results were positive in 31 cases (83.8%) of group 1 (T. gondii infected cases), while 6 cases (16.2%) were negative. In group II, 6 cases (20%) were detected as false positive (1 with C. parvum, 3 with G. lamblia and 2 with E. histolytica), while the other 24 cases (80%) were negative. All healthy control samples were negative (Table 2).
 |
Table 2 SAG1 Detection Using PCR, Nano-Gold ELISA, and ELISA Techniques |
Nano-Gold ELISA
The results of nanogold ELISA were positive in 89.2% of group 1, while 10.8%were negative. In group II (patients with other parasites) 10.0% were detected as false positive (2 cases with G. lamblia and 1 case with E. histolytica), while the other 90.0%were negative. All healthy control cases (group III) were negative. There was a statistically significant difference among the studied groups regarding positive and negative results (Table 2).
PCR
When detecting Toxoplasma SAG1 gene in the blood, 36 cases (97.3%) were positive in group I (T. gondii infected cases). In group II (patients infected with other parasites) and group III (healthy control group), all samples were negative. No false-positive results were detected. Electrophoresis bands of PCR are shown in Figure 4, group I showed a negative sample in lane 1 and positive samples in lanes 2, 3, and 4 at 356 bp. Other parasites and negative control groups showed no PCR bands. There was a significant difference among the different studied groups between positive and negative results (Table 2).
Comparison Between Different Techniques
PCR results were higher than nano-gold ELISA and ELISA regarding sensitivity (97.3%versus 89.2% and 83.8% respectively); specificity (100% versus 94% and 88% respectively); PPV (100% versus 91.7% and 83.8% respectively); NPV (98% versus 92.2% and 88% respectively) and diagnostic accuracy (98.9% versus 91.95% and 86.2% respectively) (Table 3). ROC curve (Figure 5) was used for comparing the performance of PCR, nano-gold ELISA, and ELISA in the diagnosis of toxoplasmosis. There was no significant difference between nano-gold ELISA and PCR results, while there was a significant difference between ELISA and PCR (Table 4).
|
Table 3 Diagnostic Values of PCR, Nano-Gold ELISA, and ELISA |
 |
Table 4 Comparing Areas Under the ROC Curve |
|
Figure 5 ROC curve for the performance of PCR, nano-gold ELISA and ELISA in diagnosis of toxoplasmosis. |
Discussion
Toxoplasmosis in pregnancy is a serious problem that necessitates the development of a more appropriate diagnostic method.36,37 SAG1 is stage-specific, detected only in the tachyzoite stage. It is one of the most immunogenic T. gondii antigens and could represent an important indicator of active infection.38,39 The entire Toxoplasma soluble extract is used as the antigen in the majority of commercially accessible assays. However, due to the absence of a purified standardized antigen, the tests currently in use for the detection of specific anti-Toxoplasma antibodies differ in their abilities to detect anti-Toxoplasma immunoglobulins. We tried to improve the sensitivity and specificity of toxoplasmosis diagnosis. SAG 1 was used instead of the whole Toxoplasma antigen.
In the current work, the higher improvement in gold nanoparticle surface area, which encourages more antibodies to enter the antigen–antibody complex, is likely the source of the enhanced diagnostic values of ELISA using nanoparticles. Additional factors may be small size, high-binding capacity and rapid reaction. These properties allow nano-gold-based assays to be highly sensitive.40 The use of nanomaterials is capable of assisting and improving the detection of antibodies, antigens, and DNA of T. gondii, with quick and easy handling techniques, presenting good sensitivity and specificity, indicating a potential to act in the screening and diagnosis in different stages of toxoplasmosis.41
Jalallou et al used ELISA for the detection of SAG1 T. gondii using specific IgG in human sera. Sensitivity was 88.4%, and specificity was 88%.42 They did not use IgM positive sera, while in our study this antigen was surveyed with IgM-positive sera. Another survey could detect 83% of positive IgG sera by SAG1 ELISA.43 Differences in insensitivity rates between our study and other research might be related to the purification of antigens.44 Other nanoparticles (chitosan and silica gel) were used in the diagnosis of toxoplasmosis and the authors demonstrated that nano-particle ELISA outperformed traditional ELISA in terms of sensitivity and specificity.13,45
Although serological testing has been the main diagnostic for toxoplasma infection, it has many drawbacks. DNA in blood and body fluids minimizes the problems faced when using serodiagnostic assays and makes diagnosis in challenging cases easier.46 The use of PCR has made a significant improvement in both detection of acute disease in immunocompromised patients and the prenatal diagnosis of congenital toxoplasmosis.47,48 Another study reported that in asymptomatic newborns, postnatal PCR testing of the placental tissues for Toxoplasma may provide the only sign of infection. To increase the accuracy of detecting recent infection and/or fetal transmission, serological and molecular approaches should be combined.4,49 In our study, one sample was negative with PCR. Since quick processing was not possible, DNA samples were kept at −20°C before the amplification was carried out. This may be the cause of some test sensitivity lowering. On the other hand, the presence of inhibitors in the blood samples cannot be excluded despite the effective DNA extraction technique. Our results of SAG1 detection had some limitations; first, the study of SAG1-based nano-gold ELISA needs experimental skills; second, small numbers of samples were tested. Further study is needed to include a large number of confirmed T. gondii samples.
Our results showed that PCR and nano ELISA had a high specificity for T. gondii detection than traditional ELISA. Blood from people who were not infected with T. gondii, including those who had other parasitic infections, did not trigger a PCR reaction. SAG1 does not share any characteristics with other parasite antigens, which eliminates false-positive PCR results.
In conclusion, there was no significant difference between PCR and nano-gold ELISA using SAG1 regarding sensitivity and specificity. PCR and nano-gold ELISA using SAG1 appear to be effective methods for T. gondii diagnosis. We recommend nano-gold ELISA as a promising technique in the diagnosis of toxoplasmosis and survey studies. Also, using the polyclonal antibodies conjugated with gold nanoparticles as coating antibodies and conjugate antibodies in nano-gold ELISA may achieve better sensitivity and specificity.
Acknowledgments
This study was partially supported by a grant from the Program of the Japan Initiative for Global Research Network on Infectious Diseases (J-GRID, JP22wm0125004) from the Ministry of Education, Culture, Sports, Science and Technology in Japan (MEXT), and the Japan Agency for Medical Research and Development (AMED).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Dubey JP. Review of toxoplasmosis of animals and humans (second edition). Parasit Vectors. 2010;3:112.
2. Dubey JP, Darrington C, Tiao N, et al. Isolation of viable Toxoplasma gondii from tissues and feces of cats from Addis Ababa, Ethiopia. J Parasitol. 2013;99(1):56–58. doi:10.1645/GE-3229.1
3. Al Nasr I, Ahmed F, Pullishery F, El-Ashram S, Ramaiah VV. Toxoplasmosis and anti-toxoplasma effects of medicinal plant extracts—a mini-review. Asian Pac J Trop Med. 2009;9(8):730–734. doi:10.1016/j.apjtm.2016.06.012
4. El-Sayad MH, Salem AI, Fazary H, et al. Detection of toxoplasmosis in aborted women in Alexandria, Egypt using ELISA and PCR. J Parasit Dis. 2021;45(2):539–545. doi:10.1007/s12639-020-01327-0
5. Salama DB, Fereig RM, Abdelbaky HH, et al. Toxoplasma gondii and Neospora caninum antibodies in dogs and cats from Egypt and risk factor analysis. Pathogens. 2022;11:1464. doi:10.3390/pathogens11121464
6. Calabrese K, Roberto T, Ricardo S, Corte-Real S. Ocular toxoplasmosis: the role of retinal pigment epithelium migration in infection. Parasitol Res. 2004;92:467–472. doi:10.1007/s00436-003-1031-2
7. Ahmadpour E, Daryani A, Sharif M, et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J Infect Dev Ctries. 2014;8(12):1503–1510. doi:10.3855/jidc.4796
8. Zhang K, Lin G, Han Y, Li J. Serological diagnosis of toxoplasmosis and standardization. Clin.Chim.Acta. 2016;461:83–89. doi:10.1016/j.cca.2016.07.018
9. Seng S, Yokoyama M, Suzuki R, et al. Expression of SAG1 of Toxoplasma gondii in transgenic mice. Parasitol Res. 2000;86:263–269.
10. Savva D, Morris JC, Johnson JD, Holliman RE. Polymerase chain reaction for detection of Toxoplasma gondii. J Med Microbiol. 1990;32(1):25–31. doi:10.1099/00222615-32-1-25
11. Fainardi E, Cultrera R, Romani R, et al. The role of stage-specific oligonucleotide primers in providing effective laboratory support for the molecular diagnosis of reactivated Toxoplasma gondii encephalitis in patients with AIDS. J Med Microbiol. 2002;51(10):879–890. doi:10.1099/0022-1317-51-10-879
12. Cao-Milan R, Liz-Marzan LM. Gold nanoparticle conjugates: recent advances toward clinical applications. Expert Opin Drug Deliv. 2014;11(5):74152. doi:10.1517/17425247.2014.891582
13. Yarahmadi M, Fakhar M, Ebrahimzadeh MA, Chabra A, Rahimi-esboei B. The anti-giardial effectiveness of fungal and commercial chitosan against Giardia intestinalis cysts in vitro. J Parasitic Dis. 2016;40(1):75–80. doi:10.1007/s12639-014-0449-z
14. Hegazy S, Farid A, Rabae I, El-Amir A. Novel IMB-ELISA assay for rapid diagnosis of human toxoplasmosis using SAG1 antigen. J Infect Dis. 2015;68(6):474–480. doi:10.7883/yoken.JJID.2014.444
15. World Medical Association. World Medical Association Declaration of Helsinki. JAMA. 2013;310(20):2191–2194. doi:10.1001/jama.2013.281053
16. NRC. National Research Council Guidelines for the Humane Transportation of Research Animals. Washington, DC: The National Academies Press; 2006. doi:10.17226/11557
17. Foroghiparvar F, Keshavarz H, Shojaee S. Detection of Toxoplasma gondii antigens in sera and urine of experimentally infected mice by capture– ELISA. Iran J Parasitol. 2008;3(1):1–5.
18. Sheehan D, FitzGerald R. Ion-exchange chromatography. Methods Mol Biol. 1996;59:145–150. doi:10.1385/0-89603-336-8:145
19. Engvall E, Perlmann P. Enzyme-linked immunosorbent assay (ELISA) quantitative assay of immunoglobulin G. Immunochemistry. 1971;8(9):871–874. doi:10.1016/0019-2791(71)90454-X
20. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1–2):248–254. doi:10.1016/0003-2697(76)90527-3
21. Guobadia EE, Fabemi BO. The isolation of Fasciola gigantica-specific antigens and their use in the serodiagnosis of fasciolosis in sheep by the detection of circulating antigens. Vet Parasitol. 1997;68(3):269–282. doi:10.1016/s0304-4017(96)01065-5
22. McKinney MM, Parkinson A. A simple, non-chromatographic procedure to purify immunoglobulins from serum and ascites fluid. J Immunol Methods. 1987;96(2):271–278. doi:10.1016/0022-1759(87)90324-3
23. Perosa F, Carbone R, Ferrone S, Dammacco F. Purification of human immunoglobulins by sequential precipitation with caprylic acid and ammonium sulphate. J Immunol Methods. 1990;128(1):9–16. doi:10.1016/0022-1759(90)90458-8
24. Fagbemi BO. Development and characterization of a monoclonal antibody reactive with a 28 kDa protease of Fasciola gigantica. Vet Parasitol. 1995;57(4):351–356. doi:10.1016/0304-4017(94)00685-6
25. Tijssen P, Kurstak E. Highly efficient and simple methods for the preparation of peroxidase and active peroxidase-antibody conjugates for enzyme immunoassays. Anal Biochem. 1984;136(2):451–457. doi:10.1016/0003-2697(84)90243-4
26. Zhou Y, Pan F-G, Li Y-S, et al. Colloidal gold probe-based immunochromatographic assay for the rapid detection of brevetoxins in fishery product samples. Biosens Bioelectron. 2009;24(8):2744–2747. doi:10.1016/j.bios.2009.01.034
27. Mukherjee P, Bhattacharya R, Mukhopadhyay D. Gold nanoparticles bearing functional anti-cancer drug and anti-angiogenic agent: a “2 in 1” System with potential application in cancer therapeutics. J Biomed Nanotech. 2005;2(1):2224–2228. doi:10.1166/jbn.2005.016
28. Ahmed SR, Kim J, Suzuki T, Lee J, Park EY. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosens Bioelectron. 2016;85:503–508. doi:10.1016/j.bios.2016.05.050
29. Venkatesan P, Wakelin D. ELISAs for parasitologists: or lies, damned lies and ELISAs. Parasitol. 1993;9(6):228–232. doi:10.1016/0169-4758(93)90020-g
30. Al Hamshary AS, Bayoumi IR, Aly NS, et al. Evaluation of Nano-based-ELISA for Serodiagnosis of human toxoplasmosis. Benha J Appl Sci. 2020;1(5):161–167.
31. Selseleh M, Modarressi MH, Mohebali M, et al. Real-Time RT-PCR on SAG1 and BAG1 gene expression during stage conversion in immunosuppressed mice infected with Toxoplasma gondii Tehran Strain. Korean J Parasitol. 2012;50(3):199–205.
32. Witte AK, Sickha R, Mester P, Fister S, Schoder D, Rossmanith P. Essential role 722 of polymerases for assay performance. Impact of polymerase replacement in a well 723 established assay. Biomolecular. 2018;16:12–20.
33. Al-Tamemmi IA, Abdullah BH, Raisan SJ. Molecular diagnosis for surface antigens genes (SAG1, SAG2, & SAG3) of toxoplasmosis in Basrah, Iraq province. Ejbps. 2019;6(1):99–104.
34. Greenberg RS, Daniels RS, Flanders WD, Eley JW, Boring JR. Diagnostic testing. In: Medical Epidemiology. 3rd ed. New York, NY: McGraw-Hill; 1996:77–89.
35. Khothari CR. Research methodology. In: Khothari CR, editor. Methods and Techniques. 2nd ed. New Delhi: New Age International; 2004:233–250.
36. Gamble H, Carolyn D, Dubey JP. Use of recombinant antigens for detection of Toxoplasma gondii. J Parasitol. 2000;86(3):459–462.
37. Johnson AM, Roberts H, Tenter AM. Evaluation of a recombinant antigen ELISA for the diagnosis of acute toxoplasmosis and comparison with traditional antigen ELISAs. J Med Microbiol. 1992;37:404–409.
38. Chaves-Borges FA. Detection of Toxoplasma gondii soluble antigen, SAG-1(p30), antibody and immune complex in the cerebrospinal fluid of HIV positive or negative individuals. Revista do Instituto de Medicina Tropical de São Paulo. 1999;41:329–338. doi:10.1590/S0036-46651999000600001
39. Pietkiewicz H, Hiszczynska SE, Kur J, et al. Usefulness of Toxoplasma gondii recombinant antigens in serodiagnosis of human toxoplasmosis. J Clin Microbiol. 2004;42:2504–2508.
40. Moharram AH, Mansour SA, Hussein MA, Rashad M. Direct precipitation and characterization of ZnO nanoparticles. J Nanomater. 2014;716210. doi:10.1155/2014/716210
41. Assolini JP, Concato VC, Gonçalves MD, et al. Nanomedicine advances in toxoplasmosis: diagnostic, treatment, and vaccine applications. Parasitol Res. 2017;116:1603–1615. doi:10.1007/s00436-017-5458-2
42. Jalallou N, Bandepour M, Khazan H. Recombinant SAG1 antigen to detect Toxoplasma gondii specific immunoglobulin G in human Sera by ELISA test. Iran J Parasitol. 2010;5(2):1–9.
43. Aubert D, Maine GT, Villena I. Recombinant antigens to detect antigens to detect Toxoplasma gondii specific immunoglobulin G and immunoglobulin M in human sera by enzyme immunoassay. J Clin Microbiol. 2000;38:1144–1150.
44. Chen X, Li H, Rong L. High level expression and purification of immunogenic recombinant SAG1 (P30) of Toxoplasma gondiiin Escherichia coli. Pro Exp Purif. 2001;23:33–37.
45. Aly I, Taher EE, El Nain G, et al. Advantages of bioconjugated silica-coated nanoparticles as an innovative diagnosis for human toxoplasmosis. ActaTrop. 2018;177:19–24. doi:10.1016/j.actatropica.2017.09.024
46. Jafar PAS, Keshavarz H, Rezaian M, Mohebali M, Shojaee S. Rapid detection of Toxoplasma gondii antigen in experimentally infected mice by Dot-ELISA. Iran J Parasitol. 2011;6:28–33.
47. Bastien P. Molecular diagnosis of toxoplasmosis. Trans R Soc Trop Med Hyg. 2002;96:205–215. doi:10.1016/s0035-9203(02)90078-7
48. Mahmood SH, Hassani HH, Zghair KH. Detection of B1 gene of toxoplasma gondii in blood of pregnant and abortive women infected with this parasite. Iraqi J Med. 2010;8(3):42–48. doi:10.1007/s12639-013-0382-6
49. Robert-Gangneux F, Dupretz P, Yvenou C, et al. Clinical relevance of placenta examination for the diagnosis of congenital toxoplasmosis. Pediatr Infect Dis J. 2009;29:33–38. doi:10.1097/INF.0b013e3181b20ed1





