Potential Biomedical Limitations of Graphene Nanomaterials
Introduction
In the past decade, graphene family nanomaterials (GFNs) have been extensively utilized due to their unique surface chemistry and remarkable conductivity. Since their discovery, GFNs have attracted considerable attention from researchers1,2 in various fields, including biology, physics, chemistry, and medicine for further investigation.3–6In addition, the unique structure of GFNs not only makes it the thinnest known material but also imparts exceptional physical and chemical properties to graphene.7 As a result, GFNs have a wide range of potential applications, such as circuit elements and coating materials8–12 and have received considerable attention in areas such as inks,13,14 3D printing,15,16 composite, and others.17–19 The extensive use of graphene has also stimulated the development of graphene-derived materials (Figure 1).
|
Figure 1 GFNs in biomedical, 3D printing, composite, ink and coating materials. |
The use of nanoparticles is a multi-disciplinary area of research and application, encompassing diverse fields such as physics,20 chemistry,21 materials science,22 agriculture,23 and medicine.24 Nanoparticles have attracted considerable interest among scientists due to their ability to be freely designed, their high yield, and their potential to be customized to possess specific efficacies.25 As a result, the development and research of nanoparticles have provided both theoretical support and practical opportunities for the drug delivery system (DDS) in the human body. The integration of nanoparticles in the fields of biochemistry and medicine has led to the emergence of a new interdisciplinary field focused on nanoparticle research in biomedicine. There has been an increase in the number of researchers involved in this emerging field, which has made significant progress in diverse areas such as vaccine production,26 drug delivery,27 and anti-cancer research and development.28 Of particular interest to researchers are the new materials based on carbon nanostructures,29 due to their large surface area and photothermal effect, which have been widely used in other fields. Among carbon nanostructures, research on graphene family nanomaterials in the biomedical field is increasing every year.30–37
In the last decade, numerous researchers have identified and reported applications of innovative nanomaterials in the biomedical field. Among these applications, GNFs have been used in drug delivery systems,38–41 biomedical sensing,42–45 tumor-targeted therapy,46–53 tissue engineering, and other areas.54–57 Therefore, further advances in GFNs have the potential to significantly change the current landscape of biomedicine. This review article aims to provide an overview of the literature to date regarding the use of GNFs in the areas of tumor therapy, drug delivery, and antibacterial activity. In addition, this paper, will analyze the potential risks of GNFs to the human body by collating the existing literature. Specifically, this study will analyze various GNF derivatives and their application status, the biocompatibility of GNFs with the human body, the cytotoxicity of GNFs, and their impact on human immune function.
Composition and Crystal Structures
GFNs was a class of carbon materials that are structured similarly possessing various variables, including size, layers, and surface chemistry. As shown in Figure 2, as a single atom thick sheet of two-dimensional hexagonal carbon atoms, the common GFNs includes graphene oxide (GO), reduced graphene oxide (rGO), few-layer graphene (FLG), graphene quantum dots,53 as well as downstream graphene-based nanocomposites (GBNs).54 Their remarkable mechanical properties, prominent thermal and chemical stability, temperature and radiation resistance, as well as low cost associated with high yield, further highlights its attraction for different applications.55–57 The resultant versatile and abundant carbon-based structures generated gained popularity within research, especially is expected to revolutionize the biomedical application.
 |
Figure 2 Some common graphene family nanomaterials: Graphene Oxide, reduced Graphene Oxide, Few-Layer-Graphene and Graphene-Based Nanocomposites. |
Graphene Oxide (GO) and Reduced Graphene Oxide (rGO)
Graphene oxide (GO) and reduced Graphene Oxide (rGO) hare immense potential as materials for various applications such as graphene-based electronic, optical, chemical, energy storage, and biological applications. GO is an oxidation form of graphene. The oxidation of graphene-to-graphene oxide was first reported by Hummer et al using potassium permanganate and sodium nitrate in a concentrated sulfuric acid environment. Despite the successful synthesis of GO materials, the process produces a significant amount of toxic and hazardous gases, including nitrogen dioxide and chlorine dioxide, which can cause explosions.58
Through continuous research and improvement by various researchers, a method to produce GO at a lower reaction temperature was finally discovered. This method not only reduces the generation of toxic and harmful gases, but also makes the reaction system more stable In addition, the introduction of many oxygen-containing functional groups into the GO makes it more hydrophilic and dispersible, making it a valuable and promising material for graphene-based electronic, optical, chemical, energy storage, and biological applications.59,60
RGO is a reduced state of GO, and the reduction of oxygen-containing functional groups increases the conductivity and absorption of rGO.61 Among the three methods of prepare rGO, thermochemical reduction is the simplest and most environmentally friendly, as it does not require chemical reagents to participate in the reaction.62
Few-Layer Graphene (FLG)
The properties of Few-Layer Graphene (FLG) films, which consist of ultrathin layers of carbon atoms arranged in a hexagonal honeycomb lattice, are determined by the number of graphene layers present in a film. FLG films are typically prepared by chemical vapor deposition (CVD) and are polycrystalline in nature, consisting of multiple small graphene domains grow together.63 As the number of layers per flake increases, the unique properties of monolayer graphene decrease. For example, FLGs with five layers are considered monolayer graphene, those with ten layers are called multilayer graphene, and those with more than ten layers behave like bulk graphite. Unlike monolayer graphene, FLG has the potential to evolve into hybrid materials and heterostructures by incorporating different materials into its layered structure.64,65 Thus, FLG films hold great promise for use in micro- or even nanoscale thermal diffuser applications due to their unique properties.66
Graphene-Based Nanocomposites (GBNs)
Graphene-Based Nanocomposites (GBNs) are a class of materials that typically consist of GO or rGO as the main component, combined with other active agents through covalent or non-covalent bonds to form composites that exhibit greater versatility than the original graphene material.67–69
GBNs can serve as an effective material for dispersing and stabilizing metals and metal oxides at the nanometer scale. By fully dispersing and hybridizing these components, GBNs can exhibit superior material properties compared to the individual components.70–72 Due to their high electrocatalytic activity, excellent electrical conductivity, mechanical strength, flexibility, large specific surface area, and light weight, GBNs have the potential to store electric charge, ions, or hydrogen. As a result, GBNs have emerged as a new research hotspot in various fields such as battery development, thermoelectric conversion, energy storage, biosensing, and medicine.73–77
Biomedicine Application of GFNs
Anti-Tumor Therapy
Photodynamic therapy (PDT) is a form of light therapy in which a non-toxic photosensitive material is exposed to specific wavelengths of light. When exposed to light, the photosensitive molecules on the surface of the material produce a significant amounts of reactive oxygen species (ROS). These ROS then cause severe damage to the phospholipid molecules in the tumor cell membrane, generating new ROS through the phospholipid molecules. This mechanism allows the ROS to produce toxic phototoxicity to specific cancer cells or diseased cells, resulting in therapeutic effects.78 PDT has been clinically used in the treatment of numerous diseases and is considered a minimally invasive and minimally toxic treatment modality. As research has continued, attempts have been made to enhance the anti-cancer effects of PDT by adding various materials to the therapy. However, the results of these studies have been unsatisfactory due to excessive toxicity, insufficient UV penetration energy, or lack of stability.79–87
Researchers continue to investigate carbon nanomaterials, and some studies have found that materials such as GO and reduced-state GO can absorb near-infrared (NIR) light and convert photon energy into thermal energy for better therapeutic effects. As a result, graphene family nanomaterials (GFNs) have become the most visible materials in photothermal therapy.88–92
In some related studies, researchers modified highly hydrophilic branched polyethylene glycols (PEGs) on the surface of GFNs by adding the second-generation photosensitizer chlorin e6 (Ce6) to the system via hydrophobic interactions with π-π interactions. This process resulted in the preparation of the highly hydrophilic anticancer agent GO-PEG-Ce6 for anticancer studies via PDT.93,94 The results of these studies showed that after GO-PEG-Ce6 treatment, the hydrophobicity-related poor tumor killing effect of Ce6 was largely overcome, and Ce6 successfully accumulated in large quantities in human tumors.93 The success of GO-PEG-Ce6 has generated a great deal of scientific interest in this field worldwide.87
In this environment, the GO-PEG-folate composite has emerged as an important development. The probe has good photostability, considerable luminescence quantum yield, and excellent photothermal effects. The strong photothermal effects of GO-PEG-folate depend on specific laser wavelengths (808 nm and 980 nm). Experiments have shown that probes internalized into melanomas under dark conditions did not cause tumor cell death. However, when irradiated with 808 nm and 980 nm lasers, massive melanoma death was observed. Therefore, GO-PEG-folate nanomaterials have excellent anticancer efficacy and offer a new perspective for anticancer research (Figure 3).95.
|
Figure 3 Schematic representation of the synthesis of GO-PEG-folic acid, which promotes tumor destruction by combining photodynamic and photothermal therapy using nanomaterials. |
To improve the therapeutic efficacy of photothermal and chemotherapy treatments, PEG-GO was used as a carrier for doxorubicin (DOX).96 To improve the cancer targeting ability of combined photothermal and photodynamic therapy, porphyrin photosensitizers were loaded onto folate-bound PEG-GO. In addition, graphene/TiO2 nanohybrid composites were found to exhibit high therapeutic efficacy based on photothermal and photocatalytic therapies in addition to small molecule drugs.97
Tissue Engineering
Over the past decade, significant advances in the isolation, culture, and differentiation of various cell types from embryonic and stem cells have deepened our understanding of cell functionality. This progress in the biomedical field has the potential to accelerate the discovery and development of therapies at the cellular level based on tissue engineering principles. The development of new materials, such as hydrogels and composites, has been discovered to improve the creation of tissue engineering structures. These materials mainly act as physical supports or serve as extracellular matrix (ECM) substitutes.98 The exceptional optoelectronic properties of new nanomaterials and improved machines are attracting attention, enabling the development of tissue engineering and regenerative medicine, such as artificial tendons, artificial valves, joint replacements, and other such technologies.99 However, once in the body, these materials are known to interact with tissues in the body, particularly the immune system.98,100
In the field of regenerative medicine and tissue engineering, natural or synthetic polymeric materials, including polyethylene glycol (PEG), polycaprolactone (PCL), and poly(lactic-co-glycolic acid) (PLGA), have been studied for their ability to promote stem cell differentiation.69 The advent of nanomaterials has brought new possibilities to these fields. Recent studies have shown that nanomaterials can aid in stem cell therapy, and numerous publications on this topic have appeared in major scientific journals. Of particular interest are GO and reduced rGO in graphene-based nanomaterials due to their excellent mechanical strength and electrical conductivity, which make them well suited as tissue engineering scaffolds.69,101
Recent studies have explored the potential of GO as a material to promote stem cell differentiation. Specifically, human adipose-derived stem cells were cultured on GO in combination with gelatin hydroxyapatite (HA), resulting in enhanced cell attachment, proliferation and differentiation, as well as significantly higher levels of osteogenic marker proteins. Similarly, MC3T3-E1, a mouse cranial parietal osteoblast progenitor cell, was cultured in a GO-poly(lactic-co-glycolic acid) (PLGA)-HA nanocarrier, resulting in enhanced cell attachment and mineralization, osteogenic capacity, increased alkaline phosphatase activity, and expression of osteogenic-related genes. In addition, mouse mesenchymal stem cells were shown to express osteogenic markers after two weeks of culture on GO films under osteogenic medium culture conditions. These results suggest that GO has potential as a material for promoting osteogenic differentiation of stem cells.102–104
In addition, GFNs with their favorable conductive properties have significant potential in the field of neuronal regeneration.54 Recent studies have reported the successful combination of GFNs with mechanically rigid and flexible PCL materials, cell migration mediator and polydopamine (PDA) to enhance cell adhesion, and arginine-glycine-aspartate (RGD) to develop novel porous graphene nerve conduits. The use of such scaffolds has been shown to enhance Schwann cell adhesion, thereby facilitating axonal regeneration of injured peripheral nerves in vivo and improving electrophysiological cell conduction functions such as voltage spreading and local currents (Figure 4).105
Drug Delivery System (DDS)
Graphene-based nanomaterials, including GFNs, have gained attention in drug delivery systems (DDS) due to their excellent chemical stability, high ductility, and favorable trans-cellular ability.106–108 In particular, research has focused on the use of rGO as a carrier for anticancer drugs such as SN38, which can be coupled by modifying chemical bonds such as hydrogen bonds or hydrophobic interactions.94,109,110 In addition, GFN-polymer complexes have gained interest due to their excellent cell delivery properties and flexible design freedom.111,112 A notable example is the PEG-BPEI-rGO complex developed by Kim et al which has a high loading efficiency for doxorubicin (DOX) and can be killed by photothermally induced GO endosome disruption and the proton sponge effect of BPEI escape from endosomes. This complex exhibits enhanced cytotoxicity against cancer cells under NIR irradiation, with rGO playing a critical role in the drug delivery system (Figure 5).113
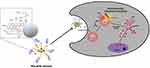 |
Figure 5 Schematic diagram of PEG-BPEI-rGO introduction into cells. |
Several studies have investigated the use of graphene oxide-PEG composites to improve material stability in physiological solutions and to treat lung and breast cancer cells (A549 and MCF-7, respectively) with the natural drug paclitaxel (PTX) loaded by hydrophobic forces. In a comparative study, the anticancer drug paclitaxel alone was used as a blank control to derive a composite drug delivery system for evaluation of anti-tumor activity. The results of the experiments showed that the combined drug delivery system group was able to rapidly enter the tumor cells and had significantly more pronounced antitumor effects with certain PTX.114
Research on rGO has demonstrated its unique role in DDS.115–120 In one study, rGO was used as a carrier to load drugs through non-covalent bonding with folic acid and doxorubicin (DOX) to achieve targeting effects and inhibit cancer cell growth.116 However, this DDS has limitations, including the system’s low targeting ability and inability to accurately detect DOX release. To address these issues, researchers have hybridized rGO with various inorganic nanoparticles to enable targeted drug delivery and drug release monitoring. The composite system loaded with DOX shows improved dispersion stability and higher drug release activity compared to DOX alone.117–120 Targeted delivery systems for anti-inflammatory drugs have also been investigated, such as loading the anti-inflammatory drug ibuprofen into a graphene oxide-chitosan system.121 In summary, GFNs have been shown to play an important role in DDS and are favored by many researchers for their ability to rapidly deliver drugs and precisely target specific areas (Table 1).
 |
Table 1 Properties of Various Types of Graphene Nanoparticles |
Other Applications of Graphene-Family Nanomaterials
GFNs have attracted considerable interest in recent years due to their unique properties such as high surface area, high electrical and thermal conductivity, and mechanical strength. The biomedical field has emerged as one of the most promising application areas for GFNs due to their potential to revolutionize drug delivery systems. While much research has been devoted to the development of GFNs for targeted drug delivery using nanoparticles, there are several other applications of GFNs worth exploring.
One such application is the use of GFNs for smart drug delivery. The concept of smart drug delivery involves the use of nanomaterials that can be programmed to release drugs in response to specific stimuli such as pH, temperature, or light. GFNs have been shown to be sensitive to environmental factors such as pH and temperature, making them ideal candidates for the development of smart drug delivery systems. In one study, GO was functionalized with a pH-responsive polymer to develop a smart drug delivery system for cancer treatment. The system was found to release the drug only in the acidic tumor microenvironment, resulting in enhanced therapeutic efficacy and reduced systemic toxicity.122
Another promising application for GFNs is transdermal drug delivery. Transdermal drug delivery systems are designed to deliver drugs through the skin, bypassing the gastrointestinal tract and liver, which can lead to drug degradation and reduced efficacy. GFNs have been shown to increase the permeability of the skin, allowing for improved drug delivery. In one study, GO was used to develop a transdermal patch for the delivery of an analgesic, resulting in improved therapeutic efficacy compared to traditional oral administration.132
Thin films and microneedles are other areas of application for GFNs in drug delivery. Thin films of GFNs can be used to coat implantable medical devices, allowing controlled drug release over an extended period of time. GFNs microneedles have also been developed for transdermal drug delivery. The high mechanical strength and flexibility of GFNs make them ideal for microneedles that require precise and controlled insertion into the skin.123
In summary, GFNs have demonstrated significant potential for use in various drug delivery applications beyond the traditional use of nanoparticles. These applications include smart drug delivery, transdermal drug delivery, thin films and microneedles. Further research in these areas is needed to fully explore the capabilities of GFNs and to develop safe and effective drug delivery systems for clinical use.
Potential Risks of Graphene Nanomaterials
Analysis of Biocompatibility and Cytotoxicity
GFNs have shown great potential for biomedical applications; however, their broad-spectrum biocompatibility and cytotoxicity need to be thoroughly analyzed before they can be widely used in the biomedical field.124–127 Studies have shown that GFNs are generally not biodegradable, especially graphene-derived materials with carboxyl group function, and some GFNs exhibit toxicity, which directly limits their uptake amount in living organisms. The analysis of possible cytotoxicity caused by nanomaterials requires consideration of the production process of preparation, purification and concentration of this material. It has been suggested that GFNs prepared by chemical vapor deposition cause an increase in the production of reactive oxygen species in neuronal cells, resulting in a significant increase in the apoptosis rate of neuronal cells.128 Studies using mice as an animal model have shown that intravenous injection of GO leads to pulmonary edema, while intraperitoneal injection of GO causes massive accumulation in the liver and spleen.125,129 GO is thought to bind to other body components, and the lungs and liver have a purifying effect on blood components, so GO is stored in large amounts in these organs. Nevertheless, GO has good biocompatibility with red blood cells, resulting in a significantly longer circulation time in the body compared to other nanomaterials.130 Furthermore, a separate study investigated the correlation between rGO and thrombosis, which showed that platelet adhesion in the experimental group was not significantly different from that in the control group, indicating that rGO has favorable hemocompatibility.131 Therefore, a comprehensive understanding of the biocompatibility and cytotoxicity of GFNs is critical for their safe and effective use in biomedical applications.
The biocompatibility of materials is a critical factor in determining their suitability for biomedical applications, with surface chemistry playing an important role. Therefore, numerous research teams have focused on improving surface chemistry and functionalities to enhance compatibility with living organisms.132–134 Yang et al demonstrated that the accumulation of nanographene sheets (NGS) in the liver could be mitigated by functionalizing them with PEG, and that the material could be gradually cleared from the body by biomass metabolism without causing significant damage to the health of mice.134 These studies have shown that modifying the surface chemistry of materials can improve their biocompatibility and that GFNs can be eliminated from the body through metabolism. Therefore, in addition to the above-mentioned methods, we recommend that the low biocompatibility and high cytotoxicity of GFNs can be addressed by coating their surface with highly biocompatible materials or by incorporating substances that promote metabolism and controlling the drug delivery dosage to a low concentration range. Such approaches can maximize the potential of GFNs for biomedical applications.
Analysis of Immunotoxicity
Evaluation of the interaction between nanomaterials and the immune system is an essential step in the development of clinical medical devices. This evaluation is particularly important for nanomaterials, such as GFNs, that are in direct contact with the body’s circulatory system. The immune system serves as a vital protective barrier for the human body. Proper immune function enables the immune system to recognize and eliminate various threats to the body’s internal environmental homeostasis, including viruses, pathogenic microorganisms, bacteria, parasites, foreign macromolecules, aging, and damaged self-cells, through a series of immune cascade responses.135,136 GFNs injected intravenously into the body enter the circulation and immediately come into contact with peripheral immune cells. This contact triggers the release of a series of cytokines, or inflammatory factors, that activate the body’s immune function. Peripheral immune cells can produce different types of cytokines, which can have pro-inflammatory and anti-inflammatory functions, depending on the specific material involved.137–148
In 2012, Sasidharan et al reported that GO significantly reduced the immunotoxicity of macrophage cell lines compared to other graphene-based materials, indicating its superior immunocompatibility.139 However, subsequent studies by Zhou et al and Li et al showed that the use of native graphene in mice resulted in significant activation of peripheral macrophages and secretion of high levels of cytokines and chemokines by Th1/Th2 immune cells, leading to cellular toxicity and induction of an inflammatory response.140,141 Changing the shape of graphene further enhanced the immunopathological responses.142 Furthermore, after injection, graphene accumulated significantly in the lungs of mice and induced the release of inflammatory factors from cells, triggering an inflammatory response and massive recruitment of macrophages and neutrophils.142,143 In addition, graphene can also stimulate Th2 cells to secrete large amounts of interleukins and soluble receptors, promoting adverse allergic reactions.144 The specific cytokines secreted have been shown to be highly dependent on the surface chemistry of the materials, leading to the development of hybrid materials with other nanoparticles to reduce specific interactions with the immune system.139,145
In addition, GO has been found to promote the differentiation of macrophages from M1 to M2 types, thereby converting them from pro-inflammatory to anti-inflammatory.146 These results suggest that GFNs materials can be used in adjuvant therapy for inflammatory responses and autoimmune diseases, but they have some limitations. For example, the production of a large amount of anti-inflammatory factors down-regulates the body’s resistance to external pathogenic microorganisms, so further modifications and designs of GO are needed.147,148 Therefore, GO modification and design of GFNs for medical use should be considered to improve their clinical applications.
In summary, GFNs have made commendable progress in regulating the immune system and mitigating immunotoxicity. Nevertheless, there is still considerable room for improvement in the future, and it is therefore imperative to be optimistic about further advances in GFNs.
Conclusion and Outlook
Since the discovery of carbon nanomaterials by Hummer et al, significant research efforts have been devoted to the study of the properties and applications of GFNs. This has led to the widespread use of carbon nanomaterials in various fields, including biomedicine. The unique two-dimensional structure and surface chemistry of GFNs enable them to adsorb a wide range of compounds, making them promising candidates for various biomedical applications, such as tumor therapy, tissue engineering, and DDS. The biocompatibility and cytotoxicity of GFNs have attracted much attention from researchers due to their direct contact with the human circulatory system. In this regard, we have summarized the specific applications of GFNs in the biomedical field and analyzed the potential risks associated with their use, based on a compilation and summary of previous literature. Furthermore, we suggest that the biocompatibility and cytotoxicity of GFNs are largely dependent on the interaction between the material and the body’s immune system. This interaction is highly correlated with the surface chemistry of the material. Therefore, complexation or drug modification of GFNs, especially GO, is a useful and feasible approach to improve their biocompatibility. Although challenges remain, there is tremendous potential for the use of GFNs in the biomedical field. With ongoing research, it is becoming increasingly possible to tailor the properties of biomaterials to meet specific needs.
Currently, numerous studies have shown that optimizing the original synthesis method of GO can improve the biocompatibility between graphene particles and cells. Surprisingly, however, several studies indicate that these improved synthesis methods not only fail to promote safe interaction between graphene particles and cells but may also exacerbate inflammatory responses between cells and tissues. This is certainly not the intended result. After carefully reviewing various articles in this field, the author believes that the inconsistencies in researchers’ choice of reaction time, different concentrations, or even different types of oxidants during the synthesis reaction could be a contributing factor. These reaction conditions could further affect the structure and characterization of the final product of GO. In addition, it is possible that different reaction times and conditions may leave different levels of impurities in the reaction product, which may affect the interaction between the product and the cell’s proteins.
The synthesis of GO is a complex process that requires precise control of various chemical reagents. A critical factor in the synthesis is the amount of chemical reagents used, which can affect the content of metal ions in the cell or cytoplasmic matrix due to subtle variations in the control of the reagents. The presence of metal ions has been closely linked to the immune response and inflammatory process of the body, which mediates the apoptosis process of cells. For example, the trace amounts of sodium sulfide in rGO have been shown to induce macrophages to release numerous inflammatory mediators, which can lead to cytokine storms and apoptosis of surrounding tissue cells. While this can have serious implications in many areas of medicine, it can also be a useful tool in tumor targeting research. Immunotherapy is a clinical approach aimed at treating tumor cells, but immunosuppressive tumors pose a significant challenge. To address this issue, some researchers have proposed the use of inflammatory responses to expose hidden antigens in tumor cells, thereby triggering the body’s immune response. Therefore, adding an appropriate amount of sodium sulfide to rGO may have a unique application in the field of tumor targeting. However, it is important to carefully control the concentration of chemical reagents during synthesis to ensure that the resulting GO particles do not cause unintended damage or inflammation.
Recent research has shown that dispersing GO materials into polymers to form composites is an effective strategy for reducing the cytotoxicity caused by GO. In addition, this dispersion improves the biocompatibility of the material by reducing the mechanical damage caused by the sharp edges of GO, which in turn provides more space for cells to adhere and interact with the material. As a result, this polymeric material has found widespread use in clinical practice, particularly in orthopedic departments. The large number of adhesion sites in the polymer system allows osteoblasts to adhere, and the presence of oxygen atoms in the GO material reduces the hydrophobicity of the composite, thereby increasing the affinity of osteoblasts for the composite. These factors make it an ideal material for orthopedic scaffolds and even orthopedic implants.
Trauma fracture patients are a common occurrence in hospitals, which underscores the clinical value of further research on graphene-related composites for orthopedic stents. In order to accelerate the translation of these findings into clinical practice, it is essential to have a thorough understanding of the biocompatibility and potential risks of graphene composites. The purpose of this article is to provide a comprehensive review of these aspects to provide a foundation for the potential applications of graphene composites.
In summary, our analysis highlights the diverse applications of graphene-based nanomaterials in various fields and presents the possibility of transforming various industries. The challenges of biocompatibility and cytotoxicity can be overcome through advances in materials science, chemistry, and immunology. Therefore, GFNs have tremendous potential for future applications.
Acknowledgment
Thanks for the funding from Key Scientific Research Projects of Institutions of Higher Learning in Henan Province (23A416007), 2018 Training Plan for Young Backbone Teachers of Institutions of Higher Learning in Henan Province (2018GGJS210) and The Henan Provincial Innovation and Entrepreneurship Training Program (S202213505010).
Disclosure
The authors declare no conflict of interest.
References
1. Novoselov KS, Geim AK, Morozov SV, et al. Electric field effect in atomically thin carbon films. Science. 2004;306(5696):666–669. doi:10.1126/science.1102896
2. Stankovich S, Dikin DA, Dommett GHB, et al. Graphene-based composite materials. Nature. 2006;442(7100):282–286. doi:10.1038/nature04969
3. Sanchez VC, Jachak A, Hurt RH, et al. Biological interactions of graphene-family nanomaterials: an interdisciplinary review. Chem. Res. Toxicol. 2012;25(1):15–34. doi:10.1021/tx200339h
4. Zhang X, Cao H, Wang H, et al. The effects of graphene-family nanomaterials on plant growth: a Review. Nanomaterials. 2022;12(6):936–951. doi:10.3390/nano12060936
5. Omran B, Baek KH. Graphene-derived antibacterial nanocomposites for water disinfection: current and future perspectives. Environ Pollut. 2022;298:118836. doi:10.1016/j.envpol.2022.118836
6. Tan C, Cao X, Wu X, et al. Recent advances in ultrathin two-dimensional nanomaterials. Chem Rev. 2017;117:6225–6331. doi:10.1021/acs.chemrev.6b00558
7. Dresselhaus MS, Araujo PT. Perspectives on the 2010 Nobel prize in physics for graphene. ACS Nano. 2010;4(11):6297–6302. doi:10.1021/nn1029789
8. Lin YM, Valdes-Garcia A, Han SJ, et al. Wafer-scale graphene integrated circuit. Science. 2011;332(6035):1294–1297. doi:10.1126/science.1204428
9. Saeed M, Palacios P, Wei MD, et al. Graphene-based microwave circuits: a Review. Adv Mater. 2021;34(48):e2108473. doi:10.1002/adma.202108473
10. Pasadas F, Feijoo PC, Mavredakis N, et al. Compact modeling technology for the simulation of integrated circuits based on graphene field-effect transistors. Adv. Mater. 2022;34(48):e2201691. doi:10.1002/adma.202201691
11. Kumar SSA, Bashir S, Ramesh K, et al. New perspectives on graphene/graphene oxide based polymer nanocomposites for corrosion applications: the relevance of the graphene/polymer barrier coatings. Prog Org Coat. 2021;154:106215. doi:10.1016/j.porgcoat.2021.106215
12. Kulyk B, Freitas MA, Santos NF, et al. A critical review on the production and application of graphene and graphene-based materials in anti-corrosion coatings. Crit Rev Solid State. 2021;47(3):309–355. doi:10.1080/10408436.2021.1886046
13. Htwe YZN, Mariatti M. Printed graphene and hybrid conductive inks for flexible, stretchable, and wearable electronics: progress, opportunities, and challenges. J Sci-Adv Mater Dev. 2022;7(1):100435.
14. Huang T, Liu W, Su C, et al. Direct ink writing of conductive materials for emerging energy storage systems. Nano Res. 2022;15(7):6091–6111. doi:10.1007/s12274-022-4200-2
15. Chiulan I, Voicu ŞI, Batalu D. The use of graphene and its Derivatives for the development of polymer matrix composites by stereolithographic 3D printing. Appl Sci. 2022;12(7):3521–3544. doi:10.3390/app12073521
16. Gaihre B, Potes MA, Serdiuk V, Tilton M, Liu X, Lu L. Two-dimensional nanomaterials-added dynamism in 3D printing and bioprinting of biomedical platforms: unique opportunities and challenges. Biomaterials. 2022;284:121507. doi:10.1016/j.biomaterials.2022.121507
17. Zhang M, Shan Y, Kong Q, et al. Applications of metal–organic framework–graphene composite materials in electrochemical energy storage. FlatChem. 2022;32:32–49. doi:10.1016/j.flatc.2021.100332
18. George JS, Vijayan P P, Paduvilan JK, et al. Advances and future outlook in epoxy/graphene composites for anticorrosive applications. Prog Org Coat. 2022;162:106571. doi:10.1016/j.porgcoat.2021.106571
19. Razaq A, Bibi F, Zheng X, et al. Review on graphene-, graphene oxide-, reduced graphene oxide-based flexible composites: from fabrication to applications. Materials. 2022;15(3):1012–1018. doi:10.3390/ma15031012
20. Hoseini-Ghahfarokhi M, Mirkiani S, Mozaffari N, et al. Applications of graphene and graphene oxide in smart drug/gene delivery: is the world still flat? Int J Nanomedicine. 2020;2020(15):9469–9496. doi:10.2147/IJN.S265876
21. Farjadian F, Abbaspour S, Sadatlu MAA, et al. Recent developments in graphene and graphene oxide: properties, synthesis, and modifications: a review. ChemistrySelect. 2020;5(33):10200–10219. doi:10.1002/slct.202002501
22. Yao Y, Dong Q, Brozena A, et al. High-entropy nanoparticles: synthesis-structure-property relationships and data-driven discovery. Science. 2022;376(6589):1–11eabn3103. doi:10.1126/science.abn3103
23. Khan N, Ali S, Latif S, et al. Biological synthesis of nanoparticles and their applications in sustainable agriculture production. Natural Science. 2022;14(06):226–234. doi:10.4236/ns.2022.146022
24. Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586(7830):516–527. doi:10.1038/s41586-020-2798-3
25. Matsuura K. Synthetic approaches to construct viral capsid-like spherical nanomaterials. Chem Commun. 2018;54(65):8944–8959. doi:10.1039/C8CC03844A
26. Cao P, Xu ZP, Li L. Tailoring functional nanoparticles for oral vaccine delivery: recent advances and future perspectives. Compos Part B-Eng. 2022;236:109826. doi:10.1016/j.compositesb.2022.109826
27. Liu R, Luo C, Pang Z, et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chinese Chem Lett. 2022;34(2):107518. doi:10.1016/j.cclet.2022.05.032
28. Liang Y, Furukawa H, Sakamoto K, et al. Anticancer activity of reconstituted ribonuclease S‐decorated artificial viral capsid. Chem Bio Chem. 2022;23(15):e202200220. doi:10.1002/cbic.202200220
29. Hajmohammadi Z, Fattahi R, Zarei-Behjani Z, et al. Carbon nanoparticles for medicine: current and future. B Mater Sci. 2021;45(1):8–26. doi:10.1007/s12034-021-02582-4
30. Dalla Colletta A, Pelin M, Sosa S, et al. CARBON-BASED nanomaterials and SKIN: an overview. Carbon. 2022;196:683–698. doi:10.1016/j.carbon.2022.05.036
31. Amiryaghoubi N, Fathi M, Barar J, et al. Recent advances in graphene-based polymer composite scaffolds for bone/cartilage tissue engineering. J Drug Deliv Sci Tec. 2022;72:103360. doi:10.1016/j.jddst.2022.103360
32. Bantun F, Singh R, Alkhanani MF, et al. Gut microbiome interactions with graphene based nanomaterials: challenges and opportunities. Sci Total Environ. 2022;830:154789. doi:10.1016/j.scitotenv.2022.154789
33. Borandeh S, Alimardani V, Abolmaali SS, et al. Graphene family nanomaterials in ocular applications: physicochemical properties and toxicity. Chem Res Toxicol. 2021;34(6):1386–1402. doi:10.1021/acs.chemrestox.0c00340
34. Wu K, Zhou Q, Ouyang S. Direct and indirect genotoxicity of graphene family nanomaterials on DNA-A review. Nanomaterials. 2021;11(11):2889–2904. doi:10.3390/nano11112889
35. Magne TM, de Oliveira Vieira T, Alencar LMR, et al. Graphene and its derivatives: understanding the main chemical and medicinal chemistry roles for biomedical applications. J Nanostructure Chem. 2021;12(5):693–727. doi:10.1007/s40097-021-00444-3
36. Kościk I, Jankowski D, Jagusiak A. Carbon Nanomaterials for Theranostic Use. C. 2021;8(1):1–16.
37. Kumar R, Rauti R, Scaini D, et al. Graphene‐based nanomaterials for neuroengineering: recent advances and future prospective. Adv Funct Mater. 2021;31(46):2104887. doi:10.1002/adfm.202104887
38. Pattnaik S, Surendra Y, Rao JV, Swain K. Carbon family nanomaterials for drug delivery applications. Nanoeng Biomater Adv Drug Delivery. 2020;2020:421–445.
39. Kulakova II, Lisichkin GV. Lisichkin, potential directions in the use of graphene nanomaterials in pharmacology and biomedicine. Pharm. Chem. J. 2022;56(1):1–11. doi:10.1007/s11094-022-02594-2
40. Yu H, Wang B, Zhou S, et al. Polyvinylpyrrolidone functionalization induces deformable structure of graphene oxide nanosheets for lung-targeting delivery. Nano Today. 2021;38:101151. doi:10.1016/j.nantod.2021.101151
41. Yi J, Choe G, Park J, et al. Graphene oxide-incorporated hydrogels for biomedical applications. Polym J. 2020;52(8):823–837. doi:10.1038/s41428-020-0350-9
42. Amiri M, Nekoueian K, Saberi RS. Graphene-family materials in electrochemical aptasensors. Anal Bioanal Chem. 2020;413(3):673–699. doi:10.1007/s00216-020-02915-y
43. Iannazzo D, Espro C, Celesti C, et al. Smart biosensors for cancer diagnosis based on graphene quantum dots. Cancers. 2021;13(13):3194–3219. doi:10.3390/cancers13133194
44. Magne TM, de Oliveira Vieira T, Costa B, et al. Factors affecting the biological response of Graphene. Colloids Surf B Biointerfaces. 2021;203:111767. doi:10.1016/j.colsurfb.2021.111767
45. Orsu P, Koyyada A. Recent progresses and challenges in graphene based nano materials for advanced therapeutical applications: a comprehensive review. Mater Today Commun. 2020;22:100823. doi:10.1016/j.mtcomm.2019.100823
46. Song S, Shen H, Wang Y, et al. Biomedical application of graphene: from drug delivery, tumor therapy, to theranostics. Colloid Surf B Biointerfaces. 2020;185:110596. doi:10.1016/j.colsurfb.2019.110596
47. Iannazzo D, Celesti C, Espro C. Recent advances on graphene quantum dots as multifunctional nanoplatforms for cancer treatment. Biotechnol J. 2021;16(2):e1900422. doi:10.1002/biot.201900422
48. Dash BS, Jose G, Lu Y-J, et al. Functionalized reduced graphene oxide as a versatile tool for cancer therapy. Int J Mol Sci. 2021;22(6):2989–3010. doi:10.3390/ijms22062989
49. Vasanthakumar A, Rejeeth C, Vivek R, et al. Design of bio-graphene-based multifunctional nanocomposites exhibits intracellular drug delivery in cervical cancer treatment. ACS Appl Bio Mater. 2022;5(6):2956–2964. doi:10.1021/acsabm.2c00280
50. Sattari S, Adeli M, Beyranvand S, et al. Functionalized graphene platforms for anticancer drug delivery. Int J Nanomedicine. 2021;16:5955–5980. doi:10.2147/IJN.S249712
51. Alemi F, Zarezadeh R, Sadigh AR, et al. Graphene oxide and reduced graphene oxide: efficient cargo platforms for cancer theranostics. J. Drug Deliv. Sci. Tec. 2020;60:101974. doi:10.1016/j.jddst.2020.101974
52. Liu J, Dong J, Zhang T, et al. Graphene-based nanomaterials and their potentials in advanced drug delivery and cancer therapy. J Control Release. 2018;286:64–73. doi:10.1016/j.jconrel.2018.07.034
53. Lin Q, Zhu Y, Wang Y, et al. Flexible quantum dot light-emitting device for emerging multifunctional and smart applications. Adv Mater. 2023:2210385. doi:10.1002/adma.202210385
54. Wang Y, Seki T, Yu X, et al. Chemistry governs water organization at a graphene electrode. Nature. 2023;1(615):1–5. doi:10.1038/s41586-022-05669-y
55. Huang K, Liu X, Lv Z, et al. MMP9-responsive graphene oxide quantum dot-based nano-in-micro drug delivery system for combinatorial therapy of choroidal neovascularization. Small. 2023:e2207335. doi:10.1002/smll.202207335
56. Kang J, Ko Y, Kim J, et al. Microwave-assisted design of nanoporous graphene membrane for ultrafast and switchable organic solvent nanofiltration. Nat Commun. 2023;14:901–913. doi:10.1038/s41467-023-36524-x
57. Guo Z, Zhang P, Xie C, et al. Defining the surface oxygen threshold that switches the interaction mode of graphene oxide with bacteria. ACS Nano. 2023. doi:10.1021/acsnano.2c10961
58. Manousi N, Rosenberg E, Deliyanni EA, Zachariadis GA. Sample preparation using graphene-oxide-derived nanomaterials for the extraction of metals. Molecules. 2020;25(10):2411–2434. doi:10.3390/molecules25102411
59. Dideikin AT, Vul AY. Graphene oxide and derivatives: the place in graphene family. Front Phys. 2019;6:149–161. doi:10.3389/fphy.2018.00149
60. Feng J, Ye Y, Xiao M, et al. Synthetic routes of the reduced graphene oxide. Chem. Pap. 2020;74(11):3767–3783. doi:10.1007/s11696-020-01196-0
61. Tarcan R, Todor-Boer O, Petrovai I, et al. Reduced graphene oxide today. J Mater Chem C. 2020;8(4):1198–1224. doi:10.1039/C9TC04916A
62. Khan M, Kumar A, Zhang J, et al. Recent advances and prospects in reduced graphene oxide-based photodetectors. J Mater Chem C. 2021;9:8129–8157. doi:10.1039/D1TC01306H
63. Wei Y, Sun Z. Liquid-phase exfoliation of graphite for mass production of pristine few-layer graphene. Curr Opin Colloid In. 2015;20(5–6):311–321. doi:10.1016/j.cocis.2015.10.010
64. Kumar V, Kumar A, Lee D-J, et al. Estimation of number of graphene layers using different methods: a focused review. Materials. 2021;14(16):4590–4611. doi:10.3390/ma14164590
65. Lv R, Robinson JA, Schaak RE, et al. Transition metal dichalcogenides and beyond: synthesis, properties, and applications of single- and few-layer nanosheets. Acc Chem Res. 2015;48(1):56–64. doi:10.1021/ar5002846
66. Chang H, Wu H. Graphene-based nanocomposites: preparation, functionalization, and energy and environmental applications. Energy Environ Sci. 2013;6(12):3483–3507. doi:10.1039/c3ee42518e
67. Ibrahim A, Klopocinska A, Horvat K, et al. Graphene-based nanocomposites: synthesis, mechanical properties, and characterizations. Polymers. 2021;13(17):2869–2900. doi:10.3390/polym13172869
68. Potts JR, Dreyer DR, Bielawski CW, et al. Graphene-based polymer nanocomposites. Polymer. 2011;52(1):5–25. doi:10.1016/j.polymer.2010.11.042
69. Lee WC, Loh KP, Lim CT. When stem cells meet graphene: opportunities and challenges in regenerative medicine. Biomaterials. 2018;155:236–250. doi:10.1016/j.biomaterials.2017.10.004
70. Khan M, Tahir MN, Adil SF, et al. Graphene based metal and metal oxide nanocomposites: synthesis, properties and their applications. J Mater Chem A. 2015;3(37):18753–18808.
71. Ji L, Meduri P, Agubra V, et al. Graphene-based nanocomposites for energy storage. Adv Energy Mater. 2016;6(16):1502159.
72. He K, Zeng Z, Chen A, et al. Advancement of Ag-graphene based nanocomposites: an overview of synthesis and its applications. Small. 2018;14(32):e1800871. doi:10.1002/smll.201800871
73. Xiang Y, Xin L, Hu J, et al. Advances in the applications of graphene-based nanocomposites in clean energy materials. Crystals. 2021;11(1):47–72. doi:10.3390/cryst11010047
74. Ma M, Li H, Xiong Y, et al. Rational design, synthesis, and application of silica/graphene-based nanocomposite: a review. Mater Design. 2021;198:109367. doi:10.1016/j.matdes.2020.109367
75. Nurazzi NM, Abdullah N, Demon SZN, et al. The frontiers of functionalized graphene-based nanocomposites as chemical sensors. Nanotechnol Rev. 2021;10(1):330–369. doi:10.1515/ntrev-2021-0030
76. Yao J, Wang H, Chen M, et al. Recent advances in graphene-based nanomaterials: properties, toxicity and applications in chemistry, biology and medicine. Microchim Acta. 2019;186(6):395–420. doi:10.1007/s00604-019-3458-x
77. Pattnaik S, Swain K, Lin Z. Graphene and graphene-based nanocomposites: biomedical applications and biosafety. J Mater Chem B. 2016;4(48):7813–7831. doi:10.1039/C6TB02086K
78. Gomes A, Neves M, Cavaleiro J. Cancer, Photodynamic therapy and porphyrin-type derivatives. An Acad Bras Cienc. 2018;90(1):993–1026. doi:10.1590/0001-3765201820170811
79. Chilakamarthi U, Giribabu L. Giribabu, photodynamic therapy: past, present and future. Chem Rec. 2017;17(8):775–802. doi:10.1002/tcr.201600121
80. Rehman FU, Zhao C, Jiang H, et al. Biomedical applications of nano-titania in theranostics and photodynamic therapy. Biomater. Sci. 2016;4(1):40–54. doi:10.1039/C5BM00332F
81. Sargazi S, Simge ER, Sacide Gelen S, et al. Application of titanium dioxide nanoparticles in photothermal and photodynamic therapy of cancer: an updated and comprehensive review. J Drug Deliv Sci Tec. 2022;75:103605. doi:10.1016/j.jddst.2022.103605
82. Cesmeli S, Biray Avci C. Application of titanium dioxide (TiO2) nanoparticles in cancer therapies. J Drug Target. 2019;27(7):762–766. doi:10.1080/1061186X.2018.1527338
83. Hou W, Shi G, Wu S, et al. Application of fullerenes as photosensitizers for antimicrobial photodynamic inactivation: a review. Front Microbiol. 2022;13:957698. doi:10.3389/fmicb.2022.957698
84. Fernandes NB, Shenoy RUK, Kajampady MK, et al. Fullerenes for the treatment of cancer: an emerging tool. Environ Sci Pollut Res Int. 2022;29(39):58607–58627. doi:10.1007/s11356-022-21449-7
85. Bucharskaya AB, Khlebtsov NG, Khlebtsov BN, et al. Photothermal and photodynamic therapy of tumors with plasmonic nanoparticles: challenges and prospects. Materials. 2022;15(4):1606–1645. doi:10.3390/ma15041606
86. Alle M, Sharma G, Lee S-H, et al. Next-generation engineered nanogold for multimodal cancer therapy and imaging: a clinical perspectives. J. Nanobiotechnol. 2022;20(1):222–256. doi:10.1186/s12951-022-01402-z
87. Youssef Z, Vanderesse R, Colombeau L, et al. The application of titanium dioxide, zinc oxide, fullerene, and graphene nanoparticles in photodynamic therapy. Cancer Nanotechnol. 2017;8(1):1606–1644. doi:10.1186/s12645-017-0032-2
88. Robinson JT, Tabakman SM, Liang Y, et al. Ultrasmall reduced graphene oxide with high near-infrared absorbance for photothermal therapy. J. Am. Chem. Soc. 2011;133(17):6825–6831. doi:10.1021/ja2010175
89. Li Y, Dong H, Li Y, et al. Graphene-based nanovehicles for photodynamic medical therapy. Int J Nanomedicine. 2015;10:2451–2459. doi:10.2147/IJN.S68600
90. Gazzi A, Fusco L, Khan A, et al. Photodynamic therapy based on graphene and MXene in cancer theranostics. Front Bioeng Biotechnol. 2019;7:295–309. doi:10.3389/fbioe.2019.00295
91. Su S, Wang J, Wei J, et al. Efficient photothermal therapy of brain cancer through porphyrin functionalized graphene oxide. New J Chem. 2015;39(7):5743–5749. doi:10.1039/C5NJ00122F
92. Li JL, Hou XL, Bao HC, et al. Graphene oxide nanoparticles for enhanced photothermal cancer cell therapy under the irradiation of a femtosecond laser beam. J Biomed Mater Res A. 2014;102(7):2181–2188. doi:10.1002/jbm.a.34871
93. Huang P, Xu C, Lin J, et al. Folic Acid-conjugated graphene oxide loaded with photosensitizers for targeting photodynamic therapy. Theranostics. 2011;1:240–250. doi:10.7150/thno/v01p0240
94. Liu Z, Robinson JT, Sun X, et al. PEGylated nanographene oxide for delivery of water-insoluble cancer drugs. J Am Chem Soc. 2008;130(33):10876–10877. doi:10.1021/ja803688x
95. Kalluru P, Vankayala R, Chiang C-S, et al. Nano-graphene oxide-mediated In vivo fluorescence imaging and bimodal photodynamic and photothermal destruction of tumors. Biomaterials. 2016;95:1–10. doi:10.1016/j.biomaterials.2016.04.006
96. Zhang W, Guo Z, Huang D, et al. Synergistic effect of chemo-photothermal therapy using PEGylated graphene oxide. Biomaterials. 2011;32(33):8555–8561. doi:10.1016/j.biomaterials.2011.07.071
97. Hu Z, Huang Y, Sun S, et al. Visible light driven photodynamic anticancer activity of graphene oxide/TiO2 hybrid. Carbon. 2012;50(3):994–1004. doi:10.1016/j.carbon.2011.10.002
98. Kharaziha M, Memic A, Akbari M, et al. Nano-enabled approaches for stem cell-based cardiac tissue engineering. Adv Healthc Mater. 2016;5(13):1533–1553. doi:10.1002/adhm.201600088
99. Ding X, Liu H, Fan Y. Graphene-based materials in regenerative medicine. Adv Healthc Mater. 2015;4(10):1451–1468. doi:10.1002/adhm.201500203
100. Paul A, Manoharan V, Krafft D, et al. Nanoengineered biomimetic hydrogels for guiding human stem cell osteogenesis in three dimensional microenvironments. J Mater Chem B. 2016;4(20):3544–3554. doi:10.1039/C5TB02745D
101. Teradal NL, Jelinek R. Carbon nanomaterials in biological studies and biomedicine. Adv Healthc Mater. 2017;6(17):700574.
102. Nair M, Nancy D, Krishnan AG, et al. Graphene oxide nanoflakes incorporated gelatin-hydroxyapatite scaffolds enhance osteogenic differentiation of human mesenchymal stem cells. Nanotechnology. 2015;26(16):161001. doi:10.1088/0957-4484/26/16/161001
103. Fu C, Bai H, Zhu J, et al. Enhanced cell proliferation and osteogenic differentiation in electrospun PLGA/hydroxyapatite nanofibre scaffolds incorporated with graphene oxide. PLoS One. 2017;12(11):e0188352. doi:10.1371/journal.pone.0188352
104. Kim J, Kim HD, Park J, et al. Enhanced osteogenic commitment of murine mesenchymal stem cells on graphene oxide substrate. Biomater Res. 2018;22:1–9. doi:10.1186/s40824-017-0112-8
105. Qian Y, Zhao X, Han Q, et al. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat Commun. 2018;9(1):323–339. doi:10.1038/s41467-017-02598-7
106. Itoo AM, Vemula SL, Gupta MT, et al. Multifunctional graphene oxide nanoparticles for drug delivery in cancer. J Control Release. 2022;350:26–59. doi:10.1016/j.jconrel.2022.08.011
107. Burdanova MG, Kharlamova MV, Kramberger C, et al. Applications of pristine and functionalized carbon nanotubes, graphene, and graphene nanoribbons in biomedicine. Nanomaterials. 2021;11(11):3020–3048. doi:10.3390/nano11113020
108. Khatik N, Sachdeva H. Graphite-based nanomaterials for drug delivery. Materialstoday Proceedings. 2022;69:30–35. doi:10.1016/j.matpr.2022.08.073
109. Li D, Zhang W, Yu X, et al. When biomolecules meet graphene: from molecular level interactions to material design and applications. Nanoscale. 2016;8(47):19491–19509. doi:10.1039/C6NR07249F
110. Oliveira AML, Machado M, Silva GA, et al. Graphene oxide thin films with drug delivery function. Nanomaterials. 2022;12(7):1149–1168. doi:10.3390/nano12071149
111. Song Z, Xu Y, Yang W, et al. Graphene/tri-block copolymer composites prepared via RAFT polymerizations for dual controlled drug delivery via pH stimulation and biodegradation. Eur Polym J. 2015;69:559–572. doi:10.1016/j.eurpolymj.2015.02.014
112. Chowdhury SM, Surhland C, Sanchez Z, et al. Graphene nanoribbons as a drug delivery agent for lucanthone mediated therapy of glioblastoma multiforme. Nanomedicine. 2015;11(1):109–118. doi:10.1016/j.nano.2014.08.001
113. Kim H, Lee D, Kim J, et al. Photothermally triggered cytosolic drug delivery via endosome disruption using a functionalized reduced graphene oxide. ACS Nano. 2013;7(8):6735–6746. doi:10.1021/nn403096s
114. Xu Z, Wang S, Li Y, et al. Covalent functionalization of graphene oxide with biocompatible poly(ethylene glycol) for delivery of paclitaxel. ACS Appl Mater Interfaces. 2014;6(19):17268–17276. doi:10.1021/am505308f
115. Singh G, Nenavathu BP. Development of rGO encapsulated polymeric beads as drug delivery system for improved loading and controlled release of doxycycline drug. Drug Dev Ind Pharm. 2020;46(3):462–470. doi:10.1080/03639045.2020.1724137
116. Park YH, Park SY, In I. Direct noncovalent conjugation of folic acid on reduced graphene oxide as anticancer drug carrier. J Ind Eng Chem. 2015;30:190–196. doi:10.1016/j.jiec.2015.05.021
117. Wang Y, Polavarapu L, Liz-Marzán LM. Reduced graphene oxide-supported gold nanostars for improved SERS sensing and drug delivery. ACS Appl Mater Interfaces. 2014;6(24):21798–21805. doi:10.1021/am501382y
118. Song J, Yang X, Jacobson O, et al. Sequential drug release and enhanced photothermal and photoacoustic effect of hybrid reduced graphene oxide-loaded ultrasmall gold nanorod vesicles for cancer therapy. ACS Nano. 2015;9(9):9199–9209. doi:10.1021/acsnano.5b03804
119. Chen H, Wang Z, Zong S, et al. SERS-fluorescence monitored drug release of a redox-responsive nanocarrier based on graphene oxide in tumor cells. ACS Appl Mater Interfaces. 2014;6(20):17526–17533. doi:10.1021/am505160v
120. Ma X, Qu Q, Zhao Y, et al. Graphene oxide wrapped gold nanoparticles for intracellular Raman imaging and drug delivery. J Mater Chem B. 2013;1(47):6495–6500. doi:10.1039/c3tb21385d
121. Perera DSM, De Silva RCL, Nayanajith LDC, et al. Anti-Inflammatory and antioxidant properties of coffea arabica/reduced graphene oxide nanocomposite prepared by green synthesis. Mater Sci Res India. 2021;18(3):305–317. doi:10.13005/msri/180306
122. Jiang C, Zhao H, Xiao H. Recent advances in graphene-family nanomaterials for effective drug delivery and phototherapy. Expert Opin Drug Deliv. 2021;18(1):119–138. doi:10.1080/17425247.2020.1798400
123. Hui Y, Yan Z, Yang H, et al. Graphene family nanomaterials for stem cell neurogenic differentiation and peripheral nerve regeneration. ACS Appl Bio Mater. 2022;5(10):4741–4759. doi:10.1021/acsabm.2c00663
124. Ou L, Song B, Liang H, et al. Toxicity of graphene-family nanoparticles: a general review of the origins and mechanisms. Part Fibre Toxicol. 2016;13(1):57–81. doi:10.1186/s12989-016-0168-y
125. Patel TN, R P, Vashi Y, et al. Toxic impacts and industrial potential of graphene. J Environ Sci Health C Toxicol Carcinog. 2020;38(3):269–297. doi:10.1080/26896583.2020.1812335
126. Xiaoli F, Qiyue C, Weihong G, et al. Toxicology data of graphene-family nanomaterials: an update. Arch Toxicol. 2020;94(6):1915–1939. doi:10.1007/s00204-020-02717-2
127. Alshehri R, Ilyas AM, Hasan A, et al. Carbon nanotubes in biomedical applications: factors, mechanisms, and remedies of toxicity. J Med Chem. 2016;59(18):8149–8167. doi:10.1021/acs.jmedchem.5b01770
128. Zhang X, Yin J, Peng C, et al. Distribution and biocompatibility studies of graphene oxide in mice after intravenous administration. Carbon. 2011;49(3):986–995. doi:10.1016/j.carbon.2010.11.005
129. Wilczek P, Major R, Lipinska L, et al. Thrombogenicity and biocompatibility studies of reduced graphene oxide modified acellular pulmonary valve tissue. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;53:310–321. doi:10.1016/j.msec.2015.04.044
130. Saleem J, Wang L, Chen C. Immunological effects of graphene family nanomaterials. NanoImpact. 2017;5:109–118. doi:10.1016/j.impact.2017.01.005
131. Li D, Hu X, Zhang S. Biodegradation of graphene-based nanomaterials in blood plasma affects their biocompatibility, drug delivery, targeted organs and antitumor ability. Biomaterials. 2019;202:12–25. doi:10.1016/j.biomaterials.2019.02.020
132. Joshi K, Mazumder B, Chattopadhyay P, et al. Graphene family of nanomaterials: reviewing advanced applications in drug delivery and medicine. Curr Drug Deliv. 2019;16(3):195–214. doi:10.2174/1567201815666181031162208
133. Wang S, Zhou L, Zheng Y, et al. Synthesis and biocompatibility of two-dimensional biomaterials. Colloid Surfaces A. 2019;583:124004. doi:10.1016/j.colsurfa.2019.124004
134. Yang K, Wan J, Zhang S, et al. In vivo pharmacokinetics, long-term biodistribution, and toxicology of PEGylated graphene in mice. ACS Nano. 2011;5(1):516–522. doi:10.1021/nn1024303
135. Parkin J, Cohen B. An overview of the immune system. Lancet. 2001;357(9270):1777–1789. doi:10.1016/S0140-6736(00)04904-7
136. Nicholson LB. The immune system. Essays Biochem. 2016;60(3):275–301. doi:10.1042/EBC20160017
137. Dudek I, Skoda M, Jarosz A, et al. The molecular influence of graphene and graphene oxide on the immune system under in vitro and in vivo conditions. Arch. Immunol. Ther. Ex. 2015;64(3):195–215. doi:10.1007/s00005-015-0369-3
138. Orecchioni M, Bedognetti D, Sgarrella F, et al. Impact of carbon nanotubes and graphene on immune cells. J Transl Med. 2014;12:138–148. doi:10.1186/1479-5876-12-138
139. Sasidharan A, Panchakarla LS, Sadanandan AR, et al. Hemocompatibility and macrophage response of pristine and functionalized graphene. Small. 2012;8(8):1251–1263. doi:10.1002/smll.201102393
140. Zhou H, Zhao K, Li W, et al. The interactions between pristine graphene and macrophages and the production of cytokines/chemokines via TLR- and NF-kappaB-related signaling pathways. Biomaterials. 2012;33(29):6933–6942. doi:10.1016/j.biomaterials.2012.06.064
141. Li Y, Zhao K, Li W, et al. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-beta signaling pathways. Biomaterials. 2012;33(2):6933–6942.
142. Schinwald A, Murphy FA, Jones A, et al. Graphene-based nanoplatelets: a new risk to the respiratory system as a consequence of their unusual aerodynamic properties. ACS Nano. 2012;6(1):736–746. doi:10.1021/nn204229f
143. Povo‐Retana A, Mojena M, Boscá A, et al. Graphene particles interfere with pro‐inflammatory polarization of human macrophages: functional and electrophysiological evidence. Adv Biol. 2021;5(11):e2100882. doi:10.1002/adbi.202100882
144. Wang X, Podila R, Shannahan JH, et al. Intravenously delivered graphene nanosheets and multiwalled carbon nanotubes induce site-specific Th2 inflammatory responses via the IL-33/ST2 axis. Int J Nanomedicine. 2013;8:1733–1748. doi:10.2147/IJN.S44211
145. Pescatori M, Bedognetti D, Venturelli E, et al. Functionalized carbon nanotubes as immunomodulator systems. Biomaterials. 2013;34(18):4395–4403. doi:10.1016/j.biomaterials.2013.02.052
146. Zolnik BS, Gonzalez-Fernandez A, Sadrieh N, et al. Nanoparticles and the immune system. Endocrinology. 2010;151(2):458–465. doi:10.1210/en.2009-1082
147. Yang Y, Asiri AM, Tang Z, et al. Graphene based materials for biomedical applications. Materials Today. 2013;16(10):365–373. doi:10.1016/j.mattod.2013.09.004
148. Hung H-S, Kung M-L, Chen F-C, et al. Nanogold-carried graphene oxide: anti-inflammation and increased differentiation capacity of mesenchymal stem cells. Nanomaterials. 2021;11(8):2046–2067. doi:10.3390/nano11082046