Stability Challenges and Engineering Strategies of Plant-Derived Extracellular Vesicle-Like Particles: A Translational Perspective
Introduction
Plant-derived extracellular vesicle-like particles (PD-EVLPs) are natural nanovesicles obtained from various plant sources.1 Terminology used to describe these vesicles has varied considerably, including nanovesicles, extracellular vesicles, exosome-like nanoparticles, and vesicle-like structures.2 According to an up-to-date consensus3 and for the sake of clarity and consistency, this review employs “PD-EVLPs” specifically to refer to vesicles isolated by plant tissue disruption, while those obtained via cell culture or vacuum extraction from plant organs are referred to as plant-derived extracellular vesicles (PDEVs).2
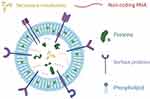
PD-EVLPs possess distinctive structural and biochemical characteristics, including a lipid bilayer encapsulating diverse bioactive molecules, such as lipids, proteins, metabolites, and nucleic acids4,5 (Figure 1). Their intricate phospholipid bilayer structure confers superior biocompatibility and in vivo stability, while the encapsulated natural bioactive constituents provide therapeutic potential.6 Collectively, these attributes render PD-EVLPs highly promising for biomedical applications, owing to their sustainability and cost-effectiveness as renewable resources derived from edible and medicinal plants,7 efficient drug delivery capabilities resulting from enhanced cellular uptake,8 intrinsic bioactivity,4,9,10 and minimal immunogenicity risks compared to animal-derived extracellular vesicles.11
|
Figure 1 The distinctive structural and biochemical characteristics of PD-EVLPs. Reprinted from Langellotto MD, Rassu G, Serri C, et al. Plant-derived extracellular vesicles: a synergetic combination of a drug delivery system and a source of natural bioactive compounds. Drug Delivery Transl Res. 2025;15(3):831–845.5 |
Despite these advantages, the stability of PD-EVLPs remains a significant yet underexplored challenge that could limit their translational potential. Studies have revealed variability in stability profiles, including aggregation tendencies,12,13 size heterogeneity, and immune responses upon administration. For instance, tea flower-derived PD-EVLPs administered intravenously in mice elevated blood C3 complement levels, suggesting immunogenicity risks.14 Conversely, PD-EVLPs enriched with phytosterols and polyphenols exhibit enhanced stability,12 indicating that their composition plays a critical role in maintaining stability.
Given these complexities, thoroughly assessing the stability of PD-EVLPs is imperative for successful clinical application. Stability variations may arise from numerous factors, including isolation methods,12,15 storage conditions,16 source plant variability,17 and administration routes.14 Therefore, this review thoroughly examines the critical factors influencing PD-EVLPs stability. Specifically, we assess current isolation and preservation techniques, investigate the molecular mechanisms underlying stability differences, examine the impact of administration routes, and explore engineering modifications to enhance PD-EVLPs stability. Through this comprehensive approach, the review aims to identify key challenges and propose strategies to optimize PD-EVLPs stability, thereby advancing their integration into biomedical research and facilitating their translational effectiveness.
Threats to PD-EVLPs Stability Posed by Isolation and Storage Conditions
The stability of PD-EVLPs is largely influenced by their isolation and storage conditions. Variations in the isolation methods, storage conditions, and media employed across different studies substantially limit the comparability and in-depth investigation of PD-EVLPs stability (Figure 2a–c and Table 1). Therefore, the establishment of standardized protocols is of great significance.
 |
Table 1 The Isolation and Preservation of PD-EVLPs |
Impact of Isolation Processes on PD-EVLPs Purity and Dispersibility, and Potential Solutions
Currently, the primary methods for isolating PD-EVLPs include ultracentrifugation, density gradient centrifugation, size exclusion chromatography (SEC), ultrafiltration, and polymer-based precipitation (eg, polyethylene glycol [PEG] precipitation).119,120 Among these, differential ultracentrifugation combined with density gradient centrifugation remains the most widely used technique at the laboratory scale due to its cost-effectiveness, high separation efficiency, high recovery rate, and suitability for large-scale sample processing.121
Techniques and Challenges in PD-EVLPs Isolation
Several PD-EVLPs isolation techniques have been developed.122 Size-exclusion chromatography (SEC) preserves vesicle integrity but requires specialized equipment, such as high-performance liquid chromatography (HPLC) systems equipped with specific columns and detectors to accurately separate and quantify nanovesicles based on their size, and is primarily suitable for small-scale, high-purity PD-EVLPs research, such as proteomic and content analyses.7 PEG precipitation reduces vesicle aggregation risks but introduces polymer contaminants, complicating subsequent purification steps.123 Meng et al exploited the natural negative charge of PD-EVLPs for separation via electrophoretic dialysis in a shorter time; however, similar to ultrafiltration,124 membrane fouling can reduce yield over time. He et al employed antibody-based immunoaffinity isolation targeting specific proteins on Arabidopsis-derived nanovesicles, achieving high-purity nanovesicles.125 However, this method is costly and requires the development of plant-specific antibodies, limiting its broad applicability. Although each method has its own merits, they remain inferior to ultracentrifugation (UC) for large-scale applications.
 |
Figure 3 General isolation process of PD-EVLPs. Reprinted from Molecular Therapy: 29/1, Dad HA, Gu TW, Zhu AQ, et al. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. 13-31, Copyright 2021 with permission from Elsevier.11 Abbreviations: PELNVs, Plant Exosome-Like Nanovesicles; UC, Ultra-Centrifugation; PBS, Phosphate Buffered Saline; AGO, Argonaute; GAPDH, Glyceraldehyde-3-Phosphate Dehydrogenase; mRNA, Messenger RNA; miRNA, MicroRNA; DNA, Deoxyribonucleic Acid; lncRNA, Long Non-coding RNA; HSP, Heat Shock Protein. |
The differential ultracentrifugation process for PD-EVLPs typically involves several steps11 (Figure 3): (1) mechanical disruption of plant materials using a juicer to obtain plant juice, followed by filtration to remove large particulate residues; (2) sequential centrifugation of the filtrate at different centrifugal forces (3000–10,000×g) at 4 °C for 10–60 minutes to remove large precipitates, cellular debris, and dead cells; (3) ultracentrifugation of the supernatant (100,000–150,000×g) for over one hour to obtain a PD-EVLPs pellet. Due to the presence of non-vesicular contaminants in the pellet, many studies further employ density gradient centrifugation to enhance PD-EVLPs purity.120,126 Despite these additional steps, vesicle-like contaminants of similar density remain, posing a threat to PD-EVLPs stability. Additionally, prolonged exposure to extreme centrifugal forces can lead to vesicle aggregation,127 potentially affecting stability.
Optimized Approaches for Improving PD-EVLPs Isolation
Concerns over nanovesicles aggregation following prolonged high-speed centrifugation have been previously raised in extracellular vesicle research. Given that PD-EVLPs are often present in higher quantities in plant extracts, aggregation may be even more pronounced. For example, Mu et al used 150,000×g centrifugation for 90 minutes, while Kim et al applied 100,000×g for 70 minutes, resulting in uneven vesicle size distribution, with some exceeding 1,000 nm in diameter, making them prone to rapid immune clearance in vivo.32,69 Chen’s research group found that optimizing centrifugation speed and duration effectively reduced aggregation and enhanced dispersibility.12 Additionally, protective agents such as trehalose have been shown to reduce vesicle size from an average of 631.4 nm to 380.4 nm following ultracentrifugation.128 However, compared to adding trehalose during the resuspension stage, introducing trehalose prematurely during the centrifugation stage may interfere with impurity removal, counteracting its stabilizing effects. Most alternative separation methods except ultracentrifugation are limited by their separation principles and costs, making it difficult to overcome the constraints of large-scale production. Thus, in the future, optimizing PD-EVLPs isolation technologies will require breakthroughs in several key areas: (1) refining ultracentrifugation parameters, particularly adjusting speed and duration, to minimize aggregation while maintaining purity and recovery rates; (2) developing alternative separation techniques, such as microfluidic-based isolation,129 to mitigate contamination risks and enhance efficiency; (3) enhancing the adaptability of standardized workflows by adjusting buffer systems and preprocessing strategies based on plant characteristics to improve PD-EVLPs stability and functionality. These optimizations will facilitate the efficient isolation and large-scale production of PD-EVLPs, while supporting their stability.
Preservation Strategies for Maintaining the Structural Integrity of PD-EVLPs
Post-isolation preservation of the structural integrity of PD‑EVLPs integrity is crucial for their clinical translation. The International Society for Extracellular Vesicles (ISEV) recommends storing extracellular vesicles (EVs) in PBS at −80 °C.130 Although most studies currently adopt −80 °C as the storage temperature and PBS as the preservation medium, whether these conditions are optimal for maintaining PD-EVLPs stability remains to be determined.
Challenges of Aggregation and Fusion During Storage
Multiple studies indicate that PD-EVLPs tend to aggregate or fuse during storage.16,131 Chen et al reported that Rehmannia glutinosa-derived extracellular vesicle-like particles (RG-EVLPs) aggregated and fused after two weeks at 4 °C or −20 °C, with further deterioration observed at all temperatures over two months.16 Even under −80 °C conditions repeated freeze-thaw cycles can compromise PD-EVLPs stability. In the study by Nemidkanam et al, Kaempferia parviflora-derived extracellular vesicle-like particles (KP-EVLPs) exhibited size increases from 300.3 ± 49.65 nm to 387.1 ± 97.53 nm after a single freeze-thaw cycle, with further enlargement to 391.9 ± 111.1 nm upon multiple cycles.74 These findings suggest that freeze-thaw cycles may induce structural alterations, potentially impairing PD-EVLPs bioactivity and drug delivery efficacy. Collectively, these results indicate that simply relying on low temperatures might not effectively protect PD-EVLPs.
Recent studies have demonstrated that formulations containing 1,3-butylene glycol (TMO) significantly enhance Dendropanax morbifera leaf-derived extracellular vesicles (LDEVs) stability at 4 °C compared to untreated LDEVs.131 Another study using depth filtration with size exclusion chromatography demonstrated that cellulose-based membranes could serve as a stable storage matrix, maintaining vesicle integrity at 4 °C.132 These findings suggest that incorporating preservatives and alternative storage matrices may broaden the potential industrial applications of PD-EVLPs by improving their storage stability.
Role of Cryoprotectants and Lyophilization
Lyophilization, a widely used method for preserving food and various biological materials, has also been applied in the clinical use of apoptotic vesicles derived from mammals.133 This approach often involves cryoprotectants to mitigate freeze-thaw damage, with mannitol demonstrating effectiveness in maintaining EV integrity during the freeze-drying process.134 Similarly the choice of cryoprotectants significantly influences extracellular vesicle stability during freezing. For example, compared to PBS alone, the addition of human serum albumin and trehalose (PBS-HAT) improves EV stability under −80 °C storage, mitigating freeze-thaw cycle damage.135 Luo et al reported that lipid vesicles preserved with trehalose maintained better size stability at −40 °C than those with sucrose, highlighting trehalose’s superior protective effects.136 Trehalose also prevents aggregation during subsequent room-temperature storage,137 although lyophilization can sometimes induce membrane damage or protein denaturation, necessitating further refinement.
Long-Term Preservation of PD-EVLPs via Optimized Technology and Source Selection
Any optimization strategy for preservation must control for plant-of-origin effects when assessing PD-EVLP stability. Some plants exhibit show stability in storage. Olea europaea L.-derived extracellular vesicle-like particles (OE-EVLPs) exhibit exceptional resilience to high temperatures (70 °C, 1 hour), a wide pH range (5–10), and mechanical stress (50–100 nm extrusion), enabling stable storage at 4°C for one month and long-term preservation with 25 mM trehalose.138 Liquid nitrogen freezing followed by 80 °C storage represents a relatively effective preservation method for PD-EVLPs. However, significant variations in preservation efficacy exist across plant species. For instance, Rehmannia glutinosa-derived nanovesicles exhibit substantial aggregation and reduced bioactivity after two months under these conditions.16 In contrast, nanovesicles from Dioscorea spp. (yam139) and Brucea javanica95 maintain stability for at least six months under the same conditions, highlighting inherent interspecies differences in PD-EVLP stability (Table 2).
 |
Table 2 Administration Methods, Particle Size and Surface Charge of PD-EVLPs |
With lyophilization already implemented in clinical applications of mammalian vesicles, refining this technique for PD-EVLPs holds great promise.133 Systematic investigation of critical parameters, including cryoprotectant formulations, drying cycles, and residual moisture control, will enable the development of standardized lyophilization protocols that maximize PD-EVLPs stability while minimizing structural damage and contamination risks. In addition, selecting plant sources with higher intrinsic stability could further improve storage feasibility, thereby promoting broader clinical and industrial applications.
Contributions of Characteristics and Composition to the Stability of PD-EVLPs
The biological functions and application potential of PD-EVLPs are closely tied to their unique structural and chemical composition. As natural drug delivery carriers, PD-EVLPs not only rely on their morphology and size for efficient in vivo distribution, but also on their membrane composition—specifically certain lipids and bioactive molecules—which directly influence their stability, targeting capability, and interactions with biological fluids.10,138
 |
Figure 4 The TEM figures of PD-EVLPs with different morphology. (a) PD-EVLPs which are spherical shape. Reprinted with permission from Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. Copyright 2021 American Chemical Society.30 (b) PD-EVLPs which are cup-shaped. Reprinted from Cai J, Pan J. Beta vulgaris-derived exosome-like nanovesicles alleviate chronic doxorubicin-induced cardiotoxicity by inhibiting ferroptosis. Journal of Biochemical and Molecular Toxicology. 2024;38(1):e23540. © 2023 Wiley Periodicals LLC. (c) PD-EVLPs which are saucer- or cup-shaped. (The scale bars in the above figures are all 100 nm) Reprinted with permission from Zeng L, Shi W, Wang H, et al. Codelivery of π-π stacked dual anticancer drugs based on aloe-derived nanovesicles for breast cancer therapy. ACS Appl. Mater. Interfaces. 2022;14(24):27686–27702. Copyright 2022 American Chemical Society.15 |
PD-EVLPs Characteristics: Protecting Its Bioactive Cargo
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) are the primary tools for analyzing the morphology and ultrastructure of PD-EVLPs. Studies have shown that PD-EVLPs exhibit diverse shapes, including spherical, disc-like, or cup-shaped structures15,30,140,168 (Figure 4). This structural diversity may be associated with their functional roles in biological systems, such as material exchange, intercellular communication, or the transport of specific bioactive molecules. Size distribution is typically analyzed using nanoparticle tracking analysis (NTA) and dynamic light scattering (DLS). Compared to PDEVs (30–150 nm in diameter) derived from cell culture supernatants, the preparation of PD-EVLPs involves mechanical disruption methods such as grinding and sonication, which directly fracture plant cell membranes and organellar membranes (including chloroplasts, vacuoles, and endoplasmic reticulum). These fragmented membranes spontaneously reassemble into vesicles through a process lacking the biological regulation inherent in conventional biogenesis of PDEVs, resulting in heterogeneous size distributions and a propensity for membrane fusion that generates larger vesicles. Consequently, PD-EVLPs typically exhibit diameters ranging from 40 to 200 nm64,169 (Figure 5a and Table 2).
 |
Figure 5 The size and surface charge of PD-EVLPs. (This figure is based on the data from Table 2) (a) The size of PD-EVLPs. (b) The surface charge of PD-EVLPs. |
In addition, the surface charge of PD-EVLPs significantly influences their biodistribution and clearance rate. In the circulatory system, cationic vesicles exhibit the fastest clearance, followed by anionic vesicles, whereas neutral or slightly negatively charged vesicles have the longest half-life.170 Due to the presence of phosphate groups on the phospholipid membrane surface of PD-EVLPs, which undergo ionization in physiological buffer environments, and the embedding of negatively charged proteins (eg, aspartic acid residues) on the membrane surface,171 most PD-EVLPs carry a net negative charge, typically exhibiting zeta potentials ranging from −30 mV to near-neutral (Figure 5b and Table 2), which helps mitigate phagocytosis by hepatic Kupffer cells, thereby extending their circulation time in vivo.172 This property provides a key advantage for PD-EVLPs as drug carriers, by ensuring their resistance to rapid clearance in the circulatory system. It is noteworthy that there are inter-study variations in both particle size and charge when investigating PD-EVLPs from the same plant (Figure 5), highlighting the necessity of establishing standardized isolation and storage protocols. Only by enhancing reproducibility and minimizing batch-to-batch variability can the clinical translation of PD-EVLPs be further advanced.
PD-EVLPs Composition: Protective Role of the Phospholipid Bilayer and Bioactive Compounds
The phospholipid bilayer of PD-EVLPs not only provides intrinsic protection for encapsulated bioactive molecules but also plays a pivotal role in maintaining vesicle stability and functionality.168 Phosphatidylcholine (PC) is a key structural component of PD-EVLPs membranes and is essential for membrane stability.21 Studies have shown that PD-EVLPs with higher PC content tend to exhibit greater stability and prolonged circulation time in vivo.173,174
The primary phospholipid composition of PD-EVLPs varies with plant source. For instance, EVLPs derived from Panax ginseng,44 aloe vera,12 and tea leaves167 exhibit high PC content. Meanwhile, tea flower-derived nanovesicles are enriched in phosphatidylserine (PS), which may reduce macrophage-mediated exosome clearance.14 Additionally, Panax ginseng-derived nanovesicles predominantly contain diglyceride monoglyceride (DGMG, 59.4%), phosphatidylethanolamine (PE, 16.8%), and ceramide (Cer, 13.8%). Studies suggest that these components affect macrophage polarization and contribute to immune regulation.151
Beyond phospholipid, PD-EVLPs are rich in phytosterols and other bioactive compounds, such as antioxidants, which regulate membrane fluidity regulation and promote ordered arrangement of phospholipids.65 Zeng et al found that aloe-derived nanovesicles, which contain aloe-emodin and β-sitosterol, exhibit superior antioxidant capacity and stability compared with synthetic liposomes (Figure 6).12 These findings suggest that the complex composition of PD-EVLPs likely underpins enhanced stability and biological functionality.
 |
Figure 6 The comparison of stability between gADNVs and liposomes. (a) The stability comparison between Liposomes and ADNVs under different conditions. (b) TEM images of gADNVs and liposomes at different storage time points. Scale bar is 100 nm. (c) Fluorescence recovery assay comparing gADNVs and liposomes. (d) Antioxidant activity of gADNVs and liposomes. ΔF/ΔF0 indicates the relative change in fluorescence intensity; *** indicates p <0.001, and the difference is statistically highly significant. Reprinted from Zeng L, Wang H, Shi W, et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J Nanobiotechnol. 2021;19(1):439.12 Abbreviations: gADNVs, Aloe-derived nanovesicles from the gel; Lips, liposomes; FL, Fluorescence. |
Building upon the understanding of PD-EVLPs’ composition-stability-function relationships, it is essential to contextualize their performance by comparing them with other widely used nanocarrier systems. PD-EVLPs, because their membrane composition, structure, and surface characteristics closely resemble those of cells, inherently possess biocompatibility and low immunogenicity. In contrast, polymer nanoparticles and liposomes, which are extensively studied nanocarriers, offer highly controllable architectures but encounter challenges such as multiple biological barriers and protein corona formation.175,176 Moreover, they lack natural bioactive molecules. Studies have shown that polymer nanoparticles can become sequestered in endosomes or lysosomes after cellular uptake, hindering effective drug release and thereby limiting drug bioavailability.177 Niosomes, known for their good stability and lower cost, are also potential candidates as nanocarriers. However, their stability depends significantly on the precise control of the cholesterol-to-surfactant ratio, and their preparation is relatively complex. Additionally, sterilization of niosomes presents challenges, as heat sterilization and membrane filtration are not suitable, potentially limiting their clinical translation.178
Elucidating Composition-Stability-Function Relationships in PD-EVLPs
Despite the promising stability and bioactivity of PD-EVLPs, their precise composition and stabilization mechanisms remain incompletely understood. Future studies should leverage high-throughput mass spectrometry and multi-omics analyses to comprehensively characterize the lipid, protein, and nucleic acid components of PD-EVLPs. Comparative studies should aim to identify key factors contributing to PD-EVLPs stability and elucidate how membrane composition influences vesicle stability, targeting properties, and drug delivery efficiency. Additionally, fluorescence tracing and in vivo imaging techniques should be employed to further investigate the distribution and degradation of PD-EVLPs under different physiological conditions, thereby enhancing their clinical feasibility. Advances in synthetic biology and nanotechnology could also facilitate PD-EVLPs engineering, such as surface ligand modifications or genetic modifications, to improve their therapeutic efficacy for specific diseases.
Disruptions to PD-EVLPs Stability Caused by Administration Routes
Different administration routes directly impact the stability, immune response, and delivery efficiency of PD-EVLPs in vivo. Oral administration allows gastrointestinal absorption and offers high safety, while intravenous injection enables rapid systemic distribution (Figure 7a). Localized delivery methods, such as transdermal patches or local injections, facilitate targeted delivery and help minimize systemic side effects.
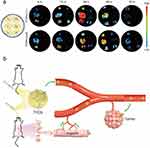 |
Figure 7 Edible tea tree flowers derived nanovesicles for drug delivery via different administration methods. (a) In vivo distribution of edible tea tree flowers derived nanovesicles under different administration methods. (b) Schematic representation and pros and cons of edible tea tree flowers derived nanovesicles under different administration methods. This article was published in Acta Pharmaceutica Sinica B, 12(2), Chen Q, Li Q, Liang Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. 907–923. Copyright Elsevier 2022.14 Abbreviations: I.v., intravenous tail; TFENs, tea flowers derived nanovesicles; Tu., tumor; He., heart; Li., liver; Sp., spleen; Lu., lung; Ki., kidney. |
Oral Administration: Overcoming Gastrointestinal Barriers for Stability and Absorption
As most PD-EVLPs originate from edible plants, oral administration is a common and practical delivery approach. Currently, many in vivo studies of PD-EVLPs have employed oral administration. Compared to intravenous injections, orally administered PD-EVLPs generally exhibit lower immunogenicity (Figure 7b).14 However, the extreme pH levels, enzymatic degradation, and mucus clearance mechanisms within the gastrointestinal tract pose significant challenges to their stability. Studies have shown that PD-EVLPs from different plant sources undergo distinct volume changes in gastrointestinal fluids. For instance, grape-derived nanovesicles shrink in size, whereas Zingiber officinale (ginger)-derived nanovesicles expand.112,156 These variations may influence the in vivo stability of PD-EVLPs; particles smaller than 10 nm are more likely to be cleared by the kidneys, while those exceeding 200 nm are more prone to phagocytosis by macrophages in the liver and spleen.179
Moreover, PD-EVLPs exhibit varying degrees of stability in the gastrointestinal environment. For example, lemon-derived nanovesicles have been shown to maintain their integrity for up to 12 hours in simulated gastric fluid.124 Certain PD-EVLPs also carry natural membrane proteins, such as heat shock proteins, which provide partial resistance to proteolytic degradation.12,174,180 Further investigation into PD-EVLPs stability and absorption mechanisms in the gastrointestinal tract will aid in optimizing oral delivery strategies. Additionally, numerous studies have shown that after oral administration, fluorescently labeled PD-EVLPs can reach various organs and exert effects, suggesting that these vesicles may withstand the harsh gastrointestinal environment.139,145,162,174,181 However, a key issue remains in determining whether the detected vesicle signals still represent intact PD-EVLPs.
Intravenous Administration: Circulatory Fate and Strategies for Prolonged Stability
Intravenous injections are widely used for PD-EVLPs delivery due to their ability to rapidly introduce vesicles into systemic circulation. However, this route exposes PD-EVLPs directly to blood components, potentially triggering recognition and clearance by the mononuclear phagocyte system (MPS). Although PD-EVLPs possess a phospholipid bilayer that generally confers high biocompatibility and low immunogenicity, their distribution and clearance rates vary significantly among different sources. For example, tea tree flower-derived nanovesicles accumulate in the liver, lungs, and tumor tissues within six hours post-injection, reaching peak concentration at 24 hours, with some vesicles still detectable after 72 hours.14 In contrast, certain PD-EVLPs, such as those derived from Catharanthus roseus180 and Panax ginseng,182 are rapidly eliminated by the liver and spleen, though the precise mechanisms governing their clearance remain unclear.
To extend PD-EVLPs circulation time and enhance therapeutic accumulation, strategies that prevent immune recognition have been explored. Blocking scavenger receptors (SRs) has been proposed to prolong blood retention and improve PD-EVLPs enrichment at tumor sites.183 However, clinical translation of this approach faces challenges, as inhibiting MPS activity may increase the risk of infections.184 Additionally, PD-EVLPs may form a protein corona in the bloodstream, which can alter their stability and biodistribution.185 Future research should focus on optimizing PD-EVLPs surface modifications to enhance circulatory stability and therapeutic efficacy.
Localized Administration: Protecting Them from the Challenge of Stability
Localized administration refers to the delivery of therapeutic agents directly to the site of injury or disease, minimizing systemic exposure and reducing side effects. This approach can be applied to both localized effects (eg, skincare and antimicrobial) and more targeted systemic effects when necessary. For example, Panax ginseng-derived nanovesicles, when injected intradermally, are efficiently internalized by skin cells within 24 hours, demonstrating effective localized delivery to the skin.154 Similarly, aloe-derived nanovesicles show strong skin penetration during non-invasive transdermal delivery, enabling targeted treatment of skin conditions.12
The high membrane fusion capability and mechanical stability of PD-EVLPs make them ideal carriers for both localized and systemic therapeutic effects. Membrane fusion capability enables these vesicles to effectively deliver therapeutic agents to the target cells by merging with cell membranes, thereby improving the bioavailability of the payload. Mechanical stability ensures that the vesicles retain their integrity during transit, protecting the encapsulated compounds from degradation and enhancing the efficacy of the treatment.186
While localized administration typically refers to direct action at the site of disease, in some cases, such as transdermal delivery, the therapeutic effect extends beyond the skin to deeper tissues. For instance, transdermal treatments for superficial nerve damage aim to achieve localized effects on the skin and underlying nerves. In contrast, direct gel implantation into the spinal cord for the treatment of spinal cord injury involves localized delivery to the site of injury, with the potential for systemic benefits, such as motor function recovery. EVLPs derived from plants like Taraxacum officinale(dandelion),187 Panax ginseng,153 and sophora188 have been incorporated into hydrogel systems, demonstrating significant antimicrobial, neuroprotective, and motor function recovery effects. The hydrogel matrix ensures a controlled and gradual release, offering potential for long-term therapeutic outcomes in both chronic disease treatment and skincare applications.
Overcoming Biological Barriers: Precision Tracking and Microenvironment-Responsive Delivery of PD-EVLPs
Tracking PD-EVLPs in vivo remains challenging, as fluorescence labeling may misinterpret stability due to vesicle degradation. More precise methods, such as isotope labeling and photoacoustic imaging, could provide a clearer picture of their integrity and biodistribution. Beyond tracking, immune clearance—particularly by the mononuclear phagocyte system (MPS), poses a significant hurdle. However, activating the immune system may not necessarily be a bad thing, as it could also trigger tumor immunity.
Additionally, surface modifications such as biomimetic coatings can enhance tissue penetration and reduce immunogenicity.189 Another key challenge is PD-EVLPs instability in acidic environments, which complicates oral delivery but could facilitate drug release in tumors. To address this, pH-responsive coatings could protect PD-EVLPs in the stomach while enabling controlled release in targeted acidic microenvironments, ultimately optimizing therapeutic efficacy.149
Optimizations in PD-EVLPs Stability Brought by Engineering Modifications
Engineering modifications aim to enhance the stability of PD-EVLPs by preventing immune recognition and optimizing surface interactions. Current mainstream strategies can be categorized into two major approaches for improving stability: biomimetic membrane camouflage and surface chemical modifications (Figure 8 and Table 3).
 |
Table 3 Strategies for Modifying PD-EVLPs |
Surface Modification to Inhibit Protein Adsorption on PD-EVLPs
Previous studies have confirmed that EVs are susceptible to plasma protein adsorption in bodily fluids, leading to the formation of a protein corona, which may accelerate their clearance and impair their targeting ability. Modulating the surface properties of PD-EVLPs, such as hydrophilicity and charge distribution, can significantly reduce nonspecific adsorption, thereby prolonging their circulation time in vivo. Among various approaches, PEG modification is the most widely used strategy. The hydrophilic polymer chains of PEG can form a dense hydration layer, generating steric hindrance that effectively prevents the adsorption of opsonins such as fibrinogen and immunoglobulins.190 For instance, PEG-modified asparagus-derived nanovesicles demonstrated a 76% reduction in blood clearance rate and a 4.3-fold increase in tumor accumulation without inducing hepatic or renal toxicity.183
However, prolonged PEG exposure may lead to the accelerated blood clearance (ABC) phenomenon, where the immune system produces anti-PEG antibodies, resulting in rapid PD-EVLPs elimination191,192 To mitigate this issue, researchers have explored alternative biocompatible molecules such as heparin to reduce immune rejection. Heparin competitively binds to the PD-EVLPs surface, creating steric hindrance that helps inhibit adsorption, prolonging circulation time. Additionally, its negative charge suppresses complement activation through electrostatic interactions.193 In a glioblastoma model, heparin-modified PD-EVLPs exhibited a 3.2-fold increase in retention time, along with a 58% reduction in serum pro-inflammatory cytokine levels (C3a/C5a)30 (Figure 9).
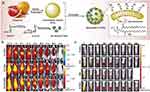 |
Figure 9 The example of prolonging the half-life of PD-EVLPs through modification. (a) The modification process of biomimetic EV-DNs. (b) Time-course in vivo fluorescence images of intracranial LN229-luc glioma-bearing mice following administration of different kinds of vesicle. (c) Time-course fluorescent imaging (IVIS) of rat serum after administration of different vesicles. Reprinted with permission from Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. Copyright 2021 American Chemical Society.30 Abbreviations: cRGD, cyclic Arg-Gly-Asp; ADH, adipic acid dihydrazide; DOX, Doxorubicin; PE, phosphatidylethanolamine. |
Surface charge modulation is another critical strategy. Introducing specific functional groups can adjust PD-EVLPs surface charge to near-neutral levels, minimizing electrostatic interactions with plasma proteins. In one study, polylysine, a positively charged polymer, was used to coat Zingiber officinale (ginger)-derived nanovesicles, successfully altering their originally negative charge.149 This demonstrated that membrane fusion-based encapsulation could effectively regulate the electrostatic properties of PD-EVLPs.
“Camouflage” Strategies for Immune Evasion and Prolonged Circulation
Biomimetic membrane coating is an emerging strategy that confers PD-EVLPs with immune-evasion capability. For example, leukocyte membrane coating leverages leukocyte integrins, such as lymphocyte function-associated antigen-1 (LFA-1), and chemokine receptors to facilitate PD-EVLPs extravasation across vascular barriers, thereby targeting inflamed or tumor sites while avoiding complement activation.189 Similarly, red blood cell (RBC) membrane coating has been widely applied in nanomedicine, because RBC membranes express cluster of differentiation 47 (CD47), a “do-not-eat-me” signal that reduces phagocytic clearance.194 The PD-EVLPs fused with exogenous membranes can be therapeutic efficacy optimized through controlled-degradation design. Yang et al demonstrated that Citrus limon-derived extracellular vesicle-like particles achieve tumor-specific enrichment by embedding into the cell membrane of tumor cells, and undergo controlled degradation under acidic tumor microenvironment conditions, thereby promoting localized and sustained drug release.195
However, cross-species membrane fusion may alter the biological characteristics of PD-EVLPs by introducing novel antigens that trigger immune rejection. Additionally, significant lipid compositional differences between plant-derived and mammalian vesicles may compromise membrane fusion stability and modification efficiency. These effects warrant further investigation.
Challenges and Future Directions in Engineering Modifications
Regardless of the modification approach used, it is crucial to consider the potential impact of these modifications on the intrinsic properties of PD-EVLPs. Changes in surface charge and modification density may affect the targeting ability of PD-EVLPs and therapeutic efficacy. Additionally, the distinct membrane compositions of plant-derived and animal-derived vesicles present a significant challenge, as many engineering strategies developed for mammalian extracellular vesicles may not be fully applicable to PD-EVLPs.
Future research should focus on evaluating the dynamic stability of PD-EVLPs in vivo and developing real-time monitoring technologies to track modification states within biological systems. Furthermore, proteomic analysis of PD-EVLPs interactions with biological components may provide new insights to optimize modification strategies. While these technologies remain largely experimental, their long-term safety and large-scale production feasibility should be further evaluated to facilitate clinical translation.
Key Considerations for Overcoming Stability Challenges
The clinical translation of PD-EVLPs hinges on overcoming a fundamental paradox: while their natural bioactivity and biocompatibility make them ideal drug carriers, their structural fragility and variability in physiological environments pose significant challenges. Stability is not a singular issue but an intricate interplay of vesicle composition, environmental interactions, and systemic processing. Addressing these challenges requires a paradigm shift—from merely optimizing storage conditions to redefining PD-EVLPs engineering by leveraging adaptive design principles, and rethinking their role within biological systems.
- Stability should not be an afterthought but an intrinsic design principle – current efforts treat PD-EVLPs stability as a post-isolation issue, focusing on storage and preservation. A more fundamental approach would be engineering PD-EVLPs at the source level—genetically modifying plants to produce vesicles with inherently higher stability and optimized bioactive cargo. Meanwhile, rigorous validation of edited PD-EVLPs (eg, multi-omics profiling and functional assays) is essential before clinical translation to ensure their biocompatibility and bioactivity. Future collaborative efforts between plant biotechnologists and nanomedicine researchers will be pivotal in systematically addressing this question.
- The immune system should be leveraged, not evaded – most modifications aim to enable PD-EVLPs to evade immune recognition. However, immune cells can be repurposed as active carriers, not just passive barriers. By exploiting the uptake of PD-EVLPs by macrophages these immune cells could serve as drug depots for sustained release within inflammatory microenvironments.
- PD-EVLPs might not need to remain intact to be effective – Stability is typically equated with structural preservation, but in some cases, controlled degradation could enhance efficacy. If PD-EVLPs could be engineered to disassemble selectively at target sites, this might improve payload release efficiency and reduce off-target effects.
- We are over-relying on extracellular vesicle paradigms from mammalian models – Many PD-EVLPs studies borrow strategies from mammalian EVs research without considering plant vesicles’ distinct lipid compositions, structural properties, and evolutionary adaptations. A tailored stability framework specific to PD-EVLPs is necessary rather than forcing them into pre-existing EV models.
- Current tracking methods create an illusion of stability – Most tracking studies rely on fluorescence labeling, assuming that detected signals represent intact PD-EVLPs. However, these signals could stem from degraded fragments, confounding stability assessments. More advanced degradation-sensitive tracking techniques are needed to truly measure PD-EVLPs integrity over time.
- Stability challenges might require a paradigm shift from single-vesicle optimization to community-level engineering – Instead of stabilizing PD-EVLPs individually, they could be designed to function cooperatively, forming vesicle networks that enhance mutual stability, similar to biofilms or viral capsid assembly.
- The goal should not just be stability, but controlled instability – The ideal PD-EVLPs should not be perfectly stable but rather exhibit “programmable degradation” tailored to different biological environments. A framework that integrates stability with controlled disassembly mechanisms could optimize PD-EVLPs performance across diverse applications.
Conclusion
PD-EVLPs show great promise as therapeutic agents and drug delivery vehicles due to their biocompatibility, bioactive cargo, and modifiability. However, stability remains a significant challenge that impedes their large-scale production, bioavailability, and clinical efficacy. Variations in lipid composition, storage conditions, and susceptibility to harsh physiological environments limit their functional lifespan. Engineering modifications, inspired by liposomal and exosomal technologies, offer potential solutions to enhance their stability. However, the long-term safety and scalability of these modifications remain areas requiring rigorous validation.
Future studies should prioritize developing standardized methods for PD-EVLP isolation, optimizing real-time monitoring of stability, and advancing targeted surface modifications to improve their functionality and lifespan. Moreover, overcoming challenges related to reproducibility, batch-to-batch variability, and large-scale production will be critical to their clinical translation. Only by ensuring both safety and stability can its potential for clinical application be validated in clinical trials. By focusing on these key areas, researchers can fully unlock the potential of PD-EVLPs for precision medicine and next-generation drug delivery.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (82173180), the Special Project of Central Government for Local Science and Technology Development of Fujian Province (2023L3011), the Joint Funds for the Innovation of Science and Technology, Fujian Province (2024Y9341), and the Startup Fund for Scientific Research, Fujian Medical University (Grant number: 2023QH2014).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li A, Li D, Gu Y, et al. Plant-derived nanovesicles: further exploration of biomedical function and application potential. Acta Pharmaceutica Sinica B. 2023;13(8):3300–3320. doi:10.1016/j.apsb.2022.12.022
2. Pinedo M, De La Canal L, De Marcos Lousa C. A call for rigor and standardization in plant extracellular vesicle research. J Extracell Vesicles. 2021;10(6):e12048. doi:10.1002/jev2.12048
3. Zhao Q, Wang T, Wang H, et al. Consensus statement on research and application of Chinese herbal medicine derived extracellular vesicles-like particles (2023 edition). Chinese Herbal Medicines. 2024;16(1):3–12. doi:10.1016/j.chmed.2023.11.002
4. Chen X, Xing X, Lin S, et al. Plant-derived nanovesicles: harnessing nature’s power for tissue protection and repair. J Nanobiotechnol. 2023;21(1):445. doi:10.1186/s12951-023-02193-7
5. Langellotto MD, Rassu G, Serri C, et al. Plant-derived extracellular vesicles: a synergetic combination of a drug delivery system and a source of natural bioactive compounds. Drug Delivery Transl Res. 2025;15(3):831–845. doi:10.1007/s13346-024-01698-4
6. Rawat S, Arora S, Dhondale MR, et al. Stability Dynamics of Plant-Based Extracellular Vesicles Drug Delivery. Journal of Xenobiotics. 2025;15(2):55. doi:10.3390/jox15020055
7. You JY, Kang SJ, Rhee WJ. Isolation of cabbage exosome-like nanovesicles and investigation of their biological activities in human cells. Bioact Mater. 2021;6(12):4321–4332. doi:10.1016/j.bioactmat.2021.04.023
8. Wang Q, Zhuang X, Mu J, et al. Delivery of therapeutic agents by nanoparticles made of grapefruit-derived lipids. Nat Commun. 2013;4(1):1867.
9. Chen X, He L, Zhang C, et al. Exploring new avenues of health protection: plant-derived nanovesicles reshape microbial communities. J Nanobiotechnol. 2024;22(1):269. doi:10.1186/s12951-024-02500-w
10. Liu H, Luo GF, Shang Z. Plant-derived nanovesicles as an emerging platform for cancer therapy. Acta Pharmaceutica Sinica B. 2024;14(1):133–154. doi:10.1016/j.apsb.2023.08.033
11. Dad HA, Gu TW, Zhu AQ, et al. Plant exosome-like nanovesicles: emerging therapeutics and drug delivery nanoplatforms. Mol Ther. 2021;29(1):13–31. doi:10.1016/j.ymthe.2020.11.030
12. Zeng L, Wang H, Shi W, et al. Aloe derived nanovesicle as a functional carrier for indocyanine green encapsulation and phototherapy. J Nanobiotechnol. 2021;19(1):439. doi:10.1186/s12951-021-01195-7
13. Chen T, Ma B, Lu S, et al. Cucumber-derived nanovesicles containing cucurbitacin B for non-small cell lung cancer therapy. Int J Nanomed. 2022;17:3583–3599. doi:10.2147/IJN.S362244
14. Chen Q, Li Q, Liang Y, et al. Natural exosome-like nanovesicles from edible tea flowers suppress metastatic breast cancer via ROS generation and microbiota modulation. Acta Pharmaceutica Sinica B. 2022;12(2):907–923. doi:10.1016/j.apsb.2021.08.016
15. Zeng L, Shi W, Wang H, et al. Codelivery of π-π stacked dual anticancer drugs based on aloe-derived nanovesicles for breast cancer therapy. ACS Appl. Mater. Interfaces. 2022;14(24):27686–27702. doi:10.1021/acsami.2c06546
16. Chen X, He L, Chen Y, et al. Evaluating stability and bioactivity of rehmannia-derived nanovesicles during storage. Sci Rep. 2024;14(1):19966. doi:10.1038/s41598-024-70334-5
17. Zhang S, Wang Q, Tan DEL, et al. Gut-liver axis: potential mechanisms of action of food-derived extracellular vesicles. J Extracell Vesicles. 2024;13(6):e12466. doi:10.1002/jev2.12466
18. Del Pozo-Acebo L, López de Las Hazas MC, Tomé-Carneiro J, et al. Therapeutic potential of broccoli-derived extracellular vesicles as nanocarriers of exogenous miRNAs. Pharmacol Res. 2022;185:106472. doi:10.1016/j.phrs.2022.106472
19. Cao Y, Hou L, Li M, et al. Broccoli extracellular vesicles enhance the therapeutic effects and restore the chemosensitivity of 5-fluorouracil on colon cancer. Food and Chemical Toxicology. 2024;186:114563. doi:10.1016/j.fct.2024.114563
20. Hossain MN, De Leo V, Tamborra R, et al. Characterization of anti-proliferative and anti-oxidant effects of nano-sized vesicles from brassica oleracea L. (broccoli). Sci Rep. 2022;12(1):14362. doi:10.1038/s41598-022-17899-1
21. Van Der Veen JN, Kennelly JP, Wan S, et al. The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim Biophys Acta Biomembr. 2017;1859(9):1558–1572. doi:10.1016/j.bbamem.2017.04.006
22. Lee BH, Wu SC, Chien HY, et al. Tomato-fruit-derived extracellular vesicles inhibit fusobacterium nucleatum via lipid-mediated mechanism. Food Funct. 2023;14(19):8942–8950. doi:10.1039/D3FO01608K
23. Daniello V, De Leo V, Lasalvia M, et al. Solanum lycopersicum (tomato)-derived nanovesicles accelerate wound healing by eliciting the migration of keratinocytes and fibroblasts. Int J Mol Sci. 2024;25(5):2452. doi:10.3390/ijms25052452
24. De Palma M, Ambrosone A, Leone A, et al. Plant roots release small extracellular vesicles with antifungal activity. Plants. 2020;9(12):1777. doi:10.3390/plants9121777
25. St-Denis-Bissonnette F, Cummings SE, Qiu S, et al. A clinically relevant large-scale biomanufacturing workflow to produce natural killer cells and natural killer cell-derived extracellular vesicles for cancer immunotherapy. J Extracell Vesicles. 2023;12(12):e12387. doi:10.1002/jev2.12387
26. Yıldırım M, Ünsal N, Kabataş B, et al. Effect of solanum lycopersicum and citrus limon-derived exosome-like vesicles on chondrogenic differentiation of adipose-derived stem cells. Appl Biochem Biotechnol. 2024;196(1):203–219. doi:10.1007/s12010-023-04491-0
27. Mammadova R, Maggio S, Fiume I, et al. Protein biocargo and anti-inflammatory effect of tomato fruit-derived nanovesicles separated by density gradient ultracentrifugation and loaded with curcumin. Pharmaceutics. 2023;15(2):333. doi:10.3390/pharmaceutics15020333
28. Kilasoniya A, Garaeva L, Shtam T, et al. Potential of plant exosome vesicles from grapefruit (citrus × paradisi) and tomato (solanum lycopersicum) juices as functional ingredients and targeted drug delivery vehicles. Antioxidants. 2023;12(4):943. doi:10.3390/antiox12040943
29. Teng Y, Mu J, Hu X, et al. Grapefruit-derived nanovectors deliver miR-18a for treatment of liver metastasis of colon cancer by induction of M1 macrophages. Oncotarget. 2016;7(18):25683–25697. doi:10.18632/oncotarget.8361
30. Niu W, Xiao Q, Wang X, et al. A biomimetic drug delivery system by integrating grapefruit extracellular vesicles and doxorubicin-loaded heparin-based nanoparticles for glioma therapy. Nano Lett. 2021;21(3):1484–1492. doi:10.1021/acs.nanolett.0c04753
31. Savcı Y, Kırbaş OK, Bozkurt BT, et al. Grapefruit-derived extracellular vesicles as a promising cell-free therapeutic tool for wound healing. Food Funct. 2021;12(11):5144–5156. doi:10.1039/D0FO02953J
32. Mu J, Zhuang X, Wang Q, et al. Interspecies communication between plant and mouse gut host cells through edible plant derived exosome-like nanoparticles. Mol Nutr Food Res. 2014;58(7):1561–1573. doi:10.1002/mnfr.201300729
33. Takakura H, Nakao T, Narita T, et al. Citrus limonL.-derived nanovesicles show an inhibitory effect on cell growth in p53-inactivated colorectal cancer cells via the macropinocytosis pathway. Biomedicines. 2022;10(6):1352. doi:10.3390/biomedicines10061352
34. Raimondo S, Urzì O, Meraviglia S, et al. Anti‐inflammatory properties of lemon‐derived extracellular vesicles are achieved through the inhibition of ERK / NF‐κB signalling pathways. J Cell & Mol Med. 2022;26(15):4195–4209. doi:10.1111/jcmm.17404
35. Zhang L, Li S, Cong M, et al. Lemon-derived extracellular vesicle-like nanoparticles block the progression of kidney stones by antagonizing endoplasmic reticulum stress in renal tubular cells. Nano Lett. 2023;23(4):1555–1563. doi:10.1021/acs.nanolett.2c05099
36. Raimondo S, Saieva L, Cristaldi M, et al. Label-free quantitative proteomic profiling of colon cancer cells identifies acetyl-CoA carboxylase alpha as antitumor target of citrus limon-derived nanovesicles. Journal of Proteomics. 2018;173:1–11. doi:10.1016/j.jprot.2017.11.017
37. Lei C, Teng Y, He L, et al. Lemon exosome-like nanoparticles enhance stress survival of gut bacteria by RNase P-mediated specific tRNA decay. Iscience. 2021;24(6):102511. doi:10.1016/j.isci.2021.102511
38. Raimondo S, Naselli F, Fontana S, et al. Citrus limon-derived nanovesicles inhibit cancer cell proliferation and suppress CML xenograft growth by inducing TRAIL-mediated cell death. Oncotarget. 2015;6(23):19514–19527. doi:10.18632/oncotarget.4004
39. Sim Y, Seo HJ, Kim DH, et al. The effect of apple-derived nanovesicles on the osteoblastogenesis of osteoblastic MC3T3-E1 cells. Journal of Medicinal Food. 2023;26(1):49–58. doi:10.1089/jmf.2022.K.0094
40. Li S, Zhang R, Wang A, et al. Panax notoginseng: derived exosome-like nanoparticles attenuate ischemia reperfusion injury via altering microglia polarization. J Nanobiotechnol. 2023;21(1):416. doi:10.1186/s12951-023-02161-1
41. Choi W, Cho JH, Park SH, et al. Ginseng root-derived exosome-like nanoparticles protect skin from UV irradiation and oxidative stress by suppressing activator protein-1 signaling and limiting the generation of reactive oxygen species. J Ginseng Res. 2024;48(2):211–219. doi:10.1016/j.jgr.2024.01.001
42. Yang L, Jin WQ, Tang XL, et al. Ginseng-derived nanoparticles inhibit lung cancer cell epithelial mesenchymal transition by repressing pentose phosphate pathway activity. Front Oncol. 2022;12:942020. doi:10.3389/fonc.2022.942020
43. Li W, Yang S, Zhao Y, et al. Ginseng-derived nanoparticles alleviate alcohol-induced liver injury by activating the Nrf2/HO-1 signalling pathway and inhibiting the NF-κB signalling pathway in vitro and in vivo. Phytomedicine. 2024;127:155428. doi:10.1016/j.phymed.2024.155428
44. Kim J, Zhu Y, Chen S, et al. Anti-glioma effect of ginseng-derived exosomes-like nanoparticles by active blood-brain-barrier penetration and tumor microenvironment modulation. J Nanobiotechnol. 2023;21(1):253. doi:10.1186/s12951-023-02006-x
45. Jang J, Jeong H, Jang E, et al. Isolation of high-purity and high-stability exosomes from ginseng. Front Plant Sci. 2023;13. doi:10.3389/fpls.2022.1064412
46. Huang J, Cao X, Wu W, et al. Investigating the proliferative inhibition of HepG2 cells by exosome-like nanovesicles derived from centella asiatica extract through metabolomics. Biomed Pharmacother. 2024;176:116855. doi:10.1016/j.biopha.2024.116855
47. Farheen J, Iqbal MZ, Lu Y, et al. Vitis vinifera kyoho-derived exosome-like nanoparticles-based drug delivery and therapeutic modalities for breast cancer therapy. J Drug Delivery Sci Technol. 2024;92:105332. doi:10.1016/j.jddst.2023.105332
48. Ipinmoroti AO, Turner J, Bellenger EJ, et al. Characterization of cannabis strain-plant-derived extracellular vesicles as potential biomarkers. Protoplasma. 2023;260(6):1603–1606. doi:10.1007/s00709-023-01870-6
49. Sundaram K, Teng Y, Mu J, et al. Outer membrane vesicles released from garlic exosome-like nanoparticles (GaELNs) train gut bacteria that reverses type 2 diabetes via the gut-brain axis. Small. 2024;20(20):e2308680. doi:10.1002/smll.202308680
50. Liu B, Li X, Yu H, et al. Therapeutic potential of garlic chive-derived vesicle-like nanoparticles in NLRP3 inflammasome-mediated inflammatory diseases. Theranostics. 2021;11(19):9311–9330. doi:10.7150/thno.60265
51. Huang XZ, Yii CY, Yong SB, et al. peu-MIR2916-p3-enriched garlic exosomes ameliorate murine colitis by reshaping gut microbiota, especially by boosting the anti-colitic bacteroides thetaiotaomicron – correspondence. Pharmacol Res. 2024;202:107131. doi:10.1016/j.phrs.2024.107131
52. Sharma V, Sinha E, Singh J. Investigation of in-vitro anti-cancer and apoptotic potential of garlic-derived nanovesicles against prostate and cervical cancer cell lines. Asian Pac J Cancer Prev. 2024;25(2):575–585. doi:10.31557/APJCP.2024.25.2.575
53. Saroj S, Us P, Patil S, et al. Herb extracellular vesicle-chitosan-PEGylated graphene oxide conjugate delivers estrogen receptor α targeting siRNA to breast cancer cells. ACS Appl Bio Mater. 2024;7(5):2741–2751. doi:10.1021/acsabm.3c01108
54. Zhang W, Song Q, Bi X, et al. Preparation of pueraria lobata root-derived exosome-like nanovesicles and evaluation of their effects on mitigating alcoholic intoxication and promoting alcohol metabolism in mice. Int J Nanomed. 2024;19:4907–4921. doi:10.2147/IJN.S462602
55. Kim JS, Kim D, Gil MC, et al. Pomegranate-derived exosome-like nanovesicles alleviate binge alcohol-induced leaky gut and liver injury. Journal of Medicinal Food. 2023;26(10):739–748. doi:10.1089/jmf.2023.K.0060
56. Sánchez-López CM, Manzaneque-López MC, Pérez-Bermúdez P, et al. Characterization and bioactivity of extracellular vesicles isolated from pomegranate. Food Funct. 2022;13(24):12870–12882. doi:10.1039/D2FO01806C
57. Jiang D, Li Z, Liu H, et al. Plant exosome-like nanovesicles derived from sesame leaves as carriers for luteolin delivery: molecular docking, stability and bioactivity. Food Chem. 2024;438:137963. doi:10.1016/j.foodchem.2023.137963
58. Hu Y, Hou Z, Liu Z, et al. Oyster mantle-derived exosomes alleviate osteoporosis by regulating bone homeostasis. Biomaterials. 2024;311:122648. doi:10.1016/j.biomaterials.2024.122648
59. Long F, Pan Y, Li J, et al. Orange-derived extracellular vesicles nanodrugs for efficient treatment of ovarian cancer assisted by transcytosis effect. Acta Pharmaceutica Sinica B. 2023;13(12):5121–5134. doi:10.1016/j.apsb.2023.04.006
60. Zhang W, Yuan Y, Li X, et al. Corrigendum: orange-derived and dexamethasone-encapsulated extracellular vesicles reduced proteinuria and alleviated pathological lesions in IgA nephropathy by targeting intestinal lymphocytes. Front Immunol. 2022;13:1047951. doi:10.3389/fimmu.2022.1047951
61. Stanly C, Moubarak M, Fiume I, et al. Membrane transporters in citrus clementina fruit juice-derived nanovesicles. Int J Mol Sci. 2019;20(24):6205. doi:10.3390/ijms20246205
62. Huang H, Yi X, Wei Q, et al. Edible and cation-free kiwi fruit derived vesicles mediated EGFR-targeted siRNA delivery to inhibit multidrug resistant lung cancer. J Nanobiotechnol. 2023;21(1):41. doi:10.1186/s12951-023-01766-w
63. Sharma S, Mahanty M, Rahaman SG, et al. Avocado‐derived extracellular vesicles loaded with ginkgetin and berberine prevent inflammation and macrophage foam cell formation. J Cell & Mol Med. 2024;28(7):e18177. doi:10.1111/jcmm.18177
64. Logozzi M, Di Raimo R, Mizzoni D, et al. Nanovesicles from organic agriculture-derived fruits and vegetables: characterization and functional antioxidant content. Int J Mol Sci. 2021;22(15):8170. doi:10.3390/ijms22158170
65. Chen C, Xia G, Zhang S, et al. Omics-based approaches for discovering active ingredients and regulating gut microbiota of actinidia arguta exosome-like nanoparticles. Food Funct. 2024;15(10):5238–5250. doi:10.1039/D3FO05783F
66. Pan Q, Bao Z, Wang Y, et al. RETRACTED: nrf2 pathway activation with natural plant-derived exosome-like nanovesicle/hydrogel preparations for oxidative stress modulation in inflammation related diseases. Chem Eng J. 2024;480:148282. doi:10.1016/j.cej.2023.148282
67. Ramírez O, Pomareda F, Olivares B, et al. Aloe vera peel-derived nanovesicles display anti-inflammatory properties and prevent myofibroblast differentiation. Phytomedicine. 2024;122:155108. doi:10.1016/j.phymed.2023.155108
68. Choi SH, Eom JY, Kim HJ, et al. Aloe-derived nanovesicles attenuate inflammation and enhance tight junction proteins for acute colitis treatment. Biomater Sci. 2023;11(16):5490–5501. doi:10.1039/D3BM00591G
69. Kim MK, Choi YC, Cho SH, et al. The antioxidant effect of small extracellular vesicles derived from aloe vera peels for wound healing. Tissue Eng and Regener Med. 2021;18(4):561–571. doi:10.1007/s13770-021-00367-8
70. Gao Q, Chen N, Li B, et al. Natural lipid nanoparticles extracted from Morus nigra L. leaves for targeted treatment of hepatocellular carcinoma via the oral route. J Nanobiotechnol. 2024;22(1):4. doi:10.1186/s12951-023-02286-3
71. Wang F, Yuan M, Shao C, et al. Momordica charantia-derived extracellular vesicles provide antioxidant protection in ulcerative colitis. Molecules. 2023;28(17):6182. doi:10.3390/molecules28176182
72. Ye L, Gao Y, Mok SWF, et al. Modulation of alveolar macrophage and mitochondrial fitness by medicinal plant-derived nanovesicles to mitigate acute lung injury and viral pneumonia. J Nanobiotechnol. 2024;22(1):190. doi:10.1186/s12951-024-02473-w
73. Nemidkanam V, Banlunara W, Chaichanawongsaroj N. Kaempferia parviflora extracellular vesicle loaded with clarithromycin for the treatment of helicobacter pylori infection. Int J Nanomed. 2024;19:1967–1983. doi:10.2147/IJN.S444686
74. Nemidkanam V, Chaichanawongsaroj N. Characterizing kaempferia parviflora extracellular vesicles, a nanomedicine candidate. PLoS One. 2022;17(1):e0262884. doi:10.1371/journal.pone.0262884
75. Emmanuela N, Muhammad DR, Iriawati N, et al. Isolation of plant-derived exosome-like nanoparticles (PDENs) from solanum nigrum L. berries and their effect on interleukin-6 expression as a potential anti-inflammatory agent. PLoS One. 2024;19(1):e0296259. doi:10.1371/journal.pone.0296259
76. Iriawati I, Vitasasti S, Rahmadian FNA, et al. Isolation and characterization of plant-derived exosome-like nanoparticles from carica papaya L. fruit and their potential as anti-inflammatory agent. PLoS One. 2024;19(7):e0304335. doi:10.1371/journal.pone.0304335
77. Zhu H, Chang M, Wang Q, et al. Identifying the potential of miRNAs in Houttuynia cordata-derived exosome-like nanoparticles against respiratory RNA viruses. Int J Nanomed. 2023;18:5983–6000. doi:10.2147/IJN.S425173
78. Yan L, Cao Y, Hou L, et al. Ginger exosome-like nanoparticle-derived miRNA therapeutics: a strategic inhibitor of intestinal inflammation. J Adv Res. 2025;69:1–15. doi:10.1016/j.jare.2024.04.001
79. Chen X, Zhou Y, Yu J. Exosome-like nanoparticles from ginger rhizomes inhibited NLRP3 inflammasome activation. Mol Pharmaceut. 2019;16(6):2690–2699. doi:10.1021/acs.molpharmaceut.9b00246
80. Zhang M, Xiao B, Wang H, et al. Edible ginger-derived nano-lipids loaded with doxorubicin as a novel drug-delivery approach for colon cancer therapy. Mol Ther. 2016;24(10):1783–1796. doi:10.1038/mt.2016.159
81. Kumar A, Sundaram K, Teng Y, et al. Ginger nanoparticles mediated induction of Foxa2 prevents high-fat diet-induced insulin resistance. Theranostics. 2022;12(3):1388–1403. doi:10.7150/thno.62514
82. Sundaram K, Miller DP, Kumar A, et al. Plant-derived exosomal nanoparticles inhibit pathogenicity of porphyromonas gingivalis. Iscience. 2019;21:308–327. doi:10.1016/j.isci.2019.10.032
83. Jokhio S, Peng I, Peng CA. Extracellular vesicles isolated from arabidopsis thaliana leaves reveal characteristics of mammalian exosomes. Protoplasma. 2024;261(5):1025–1033. doi:10.1007/s00709-024-01954-x
84. Batsukh S, Oh S, Lee JM, et al. Extracellular vesicles from ecklonia cava and phlorotannin promote rejuvenation in aged skin. Mar Drugs. 2024;22(5):223. doi:10.3390/md22050223
85. Khristiani Rahayu A, Fibriani A, Irasonia Tan M. Exploring the potential of black cumin derived nanovesicles for miRNA drug delivery. Eur J Pharm Biopharm. 2024;199:114275. doi:10.1016/j.ejpb.2024.114275
86. Zhang S, Xia J, Zhu Y, et al. Establishing salvia miltiorrhiza-derived exosome-like nanoparticles and elucidating their role in angiogenesis. Molecules. 2024;29(7):1599. doi:10.3390/molecules29071599
87. Zhu MZ, Xu HM, Liang YJ, et al. Edible exosome-like nanoparticles from portulaca oleracea L mitigate DSS-induced colitis via facilitating double-positive CD4+CD8+T cells expansion. J Nanobiotechnol. 2023;21(1):309. doi:10.1186/s12951-023-02065-0
88. Nguyen TNG, Pham CV, Chowdhury R, et al. Development of blueberry-derived extracellular nanovesicles for immunomodulatory therapy. Pharmaceutics. 2023;15(8):2115. doi:10.3390/pharmaceutics15082115
89. De Robertis M, Sarra A, D’Oria V, et al. Blueberry-derived exosome-like nanoparticles counter the response to TNF-α-induced change on gene expression in EA.hy926 cells. Biomolecules. 2020;10(5):742. doi:10.3390/biom10050742
90. Hou J, Wei W, Geng Z, et al. Developing Plant Exosomes as an Advanced Delivery System for Cosmetic Peptide. ACS Appl Bio Mater. 2024;7(5):3050–3060. doi:10.1021/acsabm.4c00096
91. Jin M, Li J, Zheng L, et al. Corosolic acid delivered by exosomes from eriobotrya japonica decreased pancreatic cancer cell proliferation and invasion by inducing SAT1-mediated ferroptosis. Int Immunopharmacol. 2024;132:111939. doi:10.1016/j.intimp.2024.111939
92. Garrett NR, Pink RC, Lawson C. Contribution of extracellular particles isolated from Morus sp. (mulberry) fruit to their reported protective health benefits: an In vitro study. Int J Mol Sci. 2024;25(11):6177. doi:10.3390/ijms25116177
93. Kusnandar MR, Wibowo I, Barlian A. Characterizing nanoparticle isolated by yam bean (pachyrhizus erosus) as a potential agent for nanocosmetics: an in vitro and in vivo approaches. Pharmaceutical Nanotechnology. 2025;13(2):341–357. doi:10.2174/0122117385279809231221050226
94. Buratta S, Latella R, Chiaradia E, et al. Characterization of nanovesicles isolated from olive vegetation water. Foods. 2024;13(6):835. doi:10.3390/foods13060835
95. Yan G, Xiao Q, Zhao J, et al. Brucea javanica derived exosome-like nanovesicles deliver miRNAs for cancer therapy. J Control Release. 2024;367:425–440. doi:10.1016/j.jconrel.2024.01.060
96. Ling Y, Li X, Gao H, et al. Biyang floral mushroom-derived exosome-like nanovesicles: characterization, absorption stability and ionizing radiation protection. Food Funct. 2024;15(13):6900–6913. doi:10.1039/D4FO00263F
97. Ishida T, Morisawa S, Jobu K, et al. Atractylodes lancea rhizome derived exosome-like nanoparticles prevent alpha-melanocyte stimulating hormone-induced melanogenesis in B16-F10 melanoma cells. Biochem Biophys Rep. 2023;35:101530. doi:10.1016/j.bbrep.2023.101530
98. Tu J, Jiang F, Fang J, et al. Anticipation and verification of dendrobium-derived nanovesicles for skin wound healing targets, predicated upon immune infiltration and senescence. Int J Nanomed. 2024;19:1629–1644. doi:10.2147/IJN.S438398
99. Vanessa V, Rachmawati H, Barlian A. Anti-inflammatory potential of goldenberry-derived exosome-like nanoparticles in macrophage polarization. Future Science OA. 2024;10(1):FSO943. doi:10.2144/fsoa-2023-0172
100. Chintapula U, Oh D, Perez C, et al. Anti‐cancer bioactivity of sweet basil leaf derived extracellular vesicles on pancreatic cancer cells. Journal of Extracellular Biology. 2024;3(2):e142. doi:10.1002/jex2.142
101. Kang M, Kang M, Lee J, et al. Allium tuberosum -derived nanovesicles with anti-inflammatory properties prevent DSS-induced colitis and modify the gut microbiome. Food Funct. 2024;15(14):7641–7657. doi:10.1039/D4FO01366B
102. Regente M, Pinedo M, San Clemente H, et al. Plant extracellular vesicles are incorporated by a fungal pathogen and inhibit its growth. Journal of Experimental Botany. 2017;68(20):5485–5495. doi:10.1093/jxb/erx355
103. Amatori S, Mazzoni L, Alvarez-Suarez JM, et al. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci Rep. 2016;6(1):30917. doi:10.1038/srep30917
104. Perut F, Roncuzzi L, Avnet S, et al. Strawberry-derived exosome-like nanoparticles prevent oxidative stress in human mesenchymal stromal cells. Biomolecules. 2021;11(1):87. doi:10.3390/biom11010087
105. Schuh CMAP, Aguayo S, Zavala G, et al. Exosome-like vesicles in Apis mellifera bee pollen, honey and royal jelly contribute to their antibacterial and pro-regenerative activity. J Exp Biol. 2019;222(Pt 20):jeb208702. doi:10.1242/jeb.208702
106. Pocsfalvi G, Turiák L, Ambrosone A, et al. Protein biocargo of citrus fruit-derived vesicles reveals heterogeneous transport and extracellular vesicle populations. Journal of Plant Physiology. 2018;229:111–121. doi:10.1016/j.jplph.2018.07.006
107. Stanly C, Alfieri M, Ambrosone A, et al. Grapefruit-derived micro and nanovesicles show distinct metabolome profiles and anticancer activities in the A375 human melanoma cell line. Cells. 2020;9(12):2722. doi:10.3390/cells9122722
108. Bruno SP, Paolini A, D’Oria V, et al. Extracellular vesicles derived from citrus sinensis modulate inflammatory genes and tight junctions in a human model of intestinal epithelium. Frontiers in Nutrition. 2021;8:778998. doi:10.3389/fnut.2021.778998
109. Liu Y, Tan ML, Zhu WJ, et al. In vitro effects of tartary buckwheat-derived nanovesicles on gut microbiota. Journal of Agricultural and Food Chemistry. 2022;70(8):2616–2629. doi:10.1021/acs.jafc.1c07658
110. Boccia E, Alfieri M, Belvedere R, et al. Plant hairy roots for the production of extracellular vesicles with antitumor bioactivity. Commun Biol. 2022;5(1):848. doi:10.1038/s42003-022-03781-3
111. Eom JY, Choi SH, Kim HJ, et al. Hemp-derived nanovesicles protect leaky gut and liver injury in dextran sodium sulfate-induced colitis. Int J Mol Sci. 2022;23(17):9955. doi:10.3390/ijms23179955
112. Liu C, Yan X, Zhang Y, et al. Oral administration of turmeric-derived exosome-like nanovesicles with anti-inflammatory and pro-resolving bioactions for murine colitis therapy. J Nanobiotechnol. 2022;20(1):206. doi:10.1186/s12951-022-01421-w
113. Kim K, Jung JH, Yoo HJ, et al. Anti-metastatic effects of plant sap-derived extracellular vesicles in a 3D microfluidic cancer metastasis model. Journal of Functional Biomaterials. 2020;11(3):49. doi:10.3390/jfb11030049
114. Lee R, Ko HJ, Kim K, et al. Anti-melanogenic effects of extracellular vesicles derived from plant leaves and stems in mouse melanoma cells and human healthy skin. J Extracell Vesicles. 2020;9(1):1703480. doi:10.1080/20013078.2019.1703480
115. Umezu T, Takanashi M, Murakami Y, et al. Acerola exosome-like nanovesicles to systemically deliver nucleic acid medicine via oral administration. Molecular Therapy. Methods & Clinical Development. 2021;21:199–208. doi:10.1016/j.omtm.2021.03.006
116. Liu B, Lu Y, Chen X, et al. Protective role of shiitake mushroom-derived exosome-like nanoparticles in D-galactosamine and lipopolysaccharide-induced acute liver injury in mice. Nutrients. 2020;12(2):477. doi:10.3390/nu12020477
117. Taşlı PN. Usage of celery root exosome as an immune suppressant; lipidomic characterization of apium graveolens originated exosomes and its suppressive effect on PMA/ionomycin mediated CD4+ T lymphocyte activation. J Food Biochem. 2022;46(12):e14393. doi:10.1111/jfbc.14393
118. Sasaki D, Kusamori K, Takayama Y, et al. Development of nanoparticles derived from corn as mass producible bionanoparticles with anticancer activity. Sci Rep. 2021;11(1):22818. doi:10.1038/s41598-021-02241-y
119. Sha A, Luo Y, Xiao W, et al. Plant-derived exosome-like nanoparticles: a comprehensive overview of their composition, biogenesis, isolation, and biological applications. Int J Mol Sci. 2024;25(22):12092. doi:10.3390/ijms252212092
120. Huang Y, Wang S, Cai Q, et al. Effective methods for isolation and purification of extracellular vesicles from plants. Journal of Integrative Plant Biology. 2021;63(12):2020–2030. doi:10.1111/jipb.13181
121. Lian MQ, Chng WH, Liang J, et al. Plant-derived extracellular vesicles: recent advancements and current challenges on their use for biomedical applications. J Extracell Vesicles. 2022;11(12):e12283. doi:10.1002/jev2.12283
122. Suresh AP, Kalarikkal SP, Pullareddy B, et al. Low pH-based method to increase the yield of plant-derived nanoparticles from fresh ginger rhizomes. ACS Omega. 2021;6(27):17635–17641. doi:10.1021/acsomega.1c02162
123. Kim M, Park JH. Isolation of aloe saponaria-derived extracellular vesicles and investigation of their potential for chronic wound healing. Pharmaceutics. 2022;14(9):1905. doi:10.3390/pharmaceutics14091905
124. Yang M, Liu X, Luo Q, et al. An efficient method to isolate lemon derived extracellular vesicles for gastric cancer therapy. J Nanobiotechnol. 2020;18(1):100. doi:10.1186/s12951-020-00656-9
125. He B, Cai Q, Qiao L, et al. RNA-binding proteins contribute to small RNA loading in plant extracellular vesicles. Nature Plants. 2021;7(3):342–352. doi:10.1038/s41477-021-00863-8
126. Bai C, Liu J, Zhang X, et al. Research status and challenges of plant-derived exosome-like nanoparticles. Biomed Pharmacother. 2024;174:116543. doi:10.1016/j.biopha.2024.116543
127. Linares R, Tan S, Gounou C, et al. High-speed centrifugation induces aggregation of extracellular vesicles. J Extracell Vesicles. 2015;4(1):29509. doi:10.3402/jev.v4.29509
128. Kocholata M, Prusova M, Auer Malinska H, et al. Comparison of two isolation methods of tobacco-derived extracellular vesicles, their characterization and uptake by plant and rat cells. Sci Rep. 2022;12(1):19896. doi:10.1038/s41598-022-23961-9
129. Lin B, Lei Y, Wang J, et al. Microfluidic-based exosome analysis for liquid biopsy. Small Methods. 2021;5(3):e2001131. doi:10.1002/smtd.202001131
130. Witwer KW, Buzás EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):20360. doi:10.3402/jev.v2i0.20360
131. Kim K, Park J, Sohn Y, et al. Stability of plant leaf-derived extracellular vesicles according to preservative and storage temperature. Pharmaceutics. 2022;14(2):457. doi:10.3390/pharmaceutics14020457
132. Pratiwi FW, Thomas RT, Karzarjeddi M, et al. Scalable purification, storage, and release of plant-derived nanovesicles for local therapy using nanostructured all-cellulose composite membranes. Biomacromolecules. 2024;25(9):5847–5859. doi:10.1021/acs.biomac.4c00535
133. Jiang Y, Li X, Huang R, et al. Lyophilized apoptotic vesicles improve hemostasis and bone regeneration in traumatic patients with impacted third molar extraction. Mol Ther. 2025:S1525001625001248. doi:10.1016/j.ymthe.2025.02.033
134. Bari E, Perteghella S, Catenacci L, et al. Freeze-dried and GMP-compliant pharmaceuticals containing exosomes for acellular mesenchymal stromal cell immunomodulant therapy. Nanomedicine. 2019;14(6):753–765. doi:10.2217/nnm-2018-0240
135. Görgens A, Corso G, Hagey DW, et al. Identification of storage conditions stabilizing extracellular vesicles preparations. J Extracell Vesicles. 2022;11(6):e12238. doi:10.1002/jev2.12238
136. Luo WC, Zhang W, Kim R, et al. Impact of controlled ice nucleation and lyoprotectants on nanoparticle stability during freeze-drying and upon storage. Int J Pharm. 2023;641:123084. doi:10.1016/j.ijpharm.2023.123084
137. Charoenviriyakul C, Takahashi Y, Nishikawa M, et al. Preservation of exosomes at room temperature using lyophilization. Int J Pharm. 2018;553(1–2):1–7. doi:10.1016/j.ijpharm.2018.10.032
138. Zhao Z, Lacombe J, Simon L, et al. Physical, biochemical, and biological characterization of olive-derived lipid nanovesicles for drug delivery applications. J Nanobiotechnol. 2024;22(1):720. doi:10.1186/s12951-024-02964-w
139. Hwang JH, Park YS, Kim HS, et al. Yam-derived exosome-like nanovesicles stimulate osteoblast formation and prevent osteoporosis in mice. J Control Release. 2023;355:184–198. doi:10.1016/j.jconrel.2023.01.071
140. Cai J, Pan J. Beta vulgaris-derived exosome-like nanovesicles alleviate chronic doxorubicin-induced cardiotoxicity by inhibiting ferroptosis. Journal of Biochemical and Molecular Toxicology. 2024;38(1):e23540. doi:10.1002/jbt.23540
141. Li C, Song Q, Yin X, et al. Preparation, characterization, and In vitro anticancer activity evaluation of broccoli-derived extracellular vesicle-coated astaxanthin nanoparticles. Molecules. 2022;27(12):3955. doi:10.3390/molecules27123955
142. Kim DK, Rhee WJ. Antioxidative effects of carrot-derived nanovesicles in cardiomyoblast and neuroblastoma cells. Pharmaceutics. 2021;13(8):1203. doi:10.3390/pharmaceutics13081203
143. Lu X, Han Q, Chen J, et al. Celery (apium graveolens L.) exosome-like nanovesicles as a new-generation chemotherapy drug delivery platform against tumor proliferation. Journal of Agricultural and Food Chemistry. 2023;71(22):8413–8424. doi:10.1021/acs.jafc.2c07760
144. Di Raimo R, Mizzoni D, Spada M, et al. Oral treatment with plant-derived exosomes restores redox balance in H2O2-treated mice. Antioxidants. 2023;12(6):1169. doi:10.3390/antiox12061169
145. Sundaram K, Mu J, Kumar A, et al. Garlic exosome-like nanoparticles reverse high-fat diet induced obesity via the gut/brain axis. Theranostics. 2022;12(3):1220–1246. doi:10.7150/thno.65427
146. Song H, Canup BSB, Ngo VL, et al. Internalization of garlic-derived nanovesicles on liver cells is triggered by interaction with CD98. ACS Omega. 2020;5(36):23118–23128. doi:10.1021/acsomega.0c02893
147. Zhuang X, Deng ZB, Mu J, et al. Ginger-derived nanoparticles protect against alcohol-induced liver damage. J Extracell Vesicles. 2015;4(1):28713. doi:10.3402/jev.v4.28713
148. Qiao Z, Zhang K, Liu J, et al. Biomimetic electrodynamic nanoparticles comprising ginger-derived extracellular vesicles for synergistic anti-infective therapy. Nat Commun. 2022;13(1):7164. doi:10.1038/s41467-022-34883-5
149. Zhang M, Yang C, Yan X, et al. Highly biocompatible functionalized layer-by-layer ginger lipid nano vectors targeting P-selectin for delivery of doxorubicin to treat colon cancer. Adv Ther. 2019;2(12):1900129. doi:10.1002/adtp.201900129
150. Sung J, Yang C, Viennois E, et al. Isolation, purification, and characterization of ginger-derived nanoparticles (GDNPs) from ginger, rhizome of zingiber officinale. Bio-Protocol. 2019;9(19):e3390. doi:10.21769/BioProtoc.3390
151. Cao M, Yan H, Han X, et al. Ginseng-derived nanoparticles alter macrophage polarization to inhibit melanoma growth. Journal for ImmunoTherapy of Cancer. 2019;7(1):326. doi:10.1186/s40425-019-0817-4
152. Tan M, Liu Y, Xu Y, et al. Plant-derived exosomes as novel nanotherapeutics contrive glycolysis reprogramming-mediated angiogenesis for diabetic ulcer healing. Biomater Res. 2024;28:35. doi:10.34133/bmr.0035
153. Xu XH, Yuan TJ, Dad HA, et al. Plant exosomes As novel nanoplatforms for MicroRNA transfer stimulate neural differentiation of stem cells In vitro and In vivo. Nano Lett. 2021;21(19):8151–8159. doi:10.1021/acs.nanolett.1c02530
154. Yang S, Lu S, Ren L, et al. Ginseng-derived nanoparticles induce skin cell proliferation and promote wound healing. J Ginseng Res. 2023;47(1):133–143. doi:10.1016/j.jgr.2022.07.005
155. Zhou X, Xu S, Zhang Z, et al. Gouqi-derived nanovesicles (GqDNVs) inhibited dexamethasone-induced muscle atrophy associating with AMPK/SIRT1/PGC1α signaling pathway. J Nanobiotechnol. 2024;22(1):276. doi:10.1186/s12951-024-02563-9
156. Rahimi Ghiasi M, Rahimi E, Amirkhani Z, et al. Leucine-rich repeat-containing G-protein coupled receptor 5 gene overexpression of the rat small intestinal progenitor cells in response to orally administered grape exosome-like nanovesicles. Adv Biomed Res. 2018;7(1):125. doi:10.4103/abr.abr_114_18
157. Feng W, Teng Y, Zhong Q, et al. Biomimetic grapefruit-derived extracellular vesicles for safe and targeted delivery of sodium thiosulfate against vascular calcification. ACS Nano. 2023;17(24):24773–24789. doi:10.1021/acsnano.3c05261
158. Chen X, Liu B, Li X, et al. Identification of anti-inflammatory vesicle-like nanoparticles in honey. J Extracell Vesicles. 2021;10(4):e12069. doi:10.1002/jev2.12069
159. Xiao Q, Zhao W, Wu C, et al. Lemon-derived extracellular vesicles nanodrugs enable to efficiently overcome cancer multidrug resistance by endocytosis-triggered energy dissipation and energy production reduction. Adv Sci. 2022;9(20):e2105274. doi:10.1002/advs.202105274
160. Cai H, Huang LY, Hong R, et al. Momordica charantia Exosome-Like Nanoparticles Exert Neuroprotective Effects Against Ischemic Brain Injury via Inhibiting Matrix Metalloproteinase 9 and Activating the AKT/GSK3β Signaling Pathway. Front Pharmacol. 2022;13:908830. doi:10.3389/fphar.2022.908830
161. Pomatto MAC, Gai C, Negro F, et al. Plant-derived extracellular vesicles as a delivery platform for RNA-based vaccine: feasibility study of an oral and intranasal SARS-CoV-2 vaccine. Pharmaceutics. 2023;15(3):974. doi:10.3390/pharmaceutics15030974
162. Berger E, Colosetti P, Jalabert A, et al. Use of nanovesicles from Orange juice to reverse diet-induced gut modifications in diet-induced obese mice. Mol Ther Meth Clin Develop. 2020;18:880–892. doi:10.1016/j.omtm.2020.08.009
163. Zhan W, Deng M, Huang X, et al. Pueraria lobata-derived exosome-like nanovesicles alleviate osteoporosis by enhacning autophagy. J Control Release. 2023;364:644–653. doi:10.1016/j.jconrel.2023.11.020
164. Sasaki D, Suzuki H, Kusamori K, et al. Development of rice bran-derived nanoparticles with excellent anti-cancer activity and their application for peritoneal dissemination. J Nanobiotechnol. 2024;22(1):114. doi:10.1186/s12951-024-02381-z
165. Teng Y, Xu F, Zhang X, et al. Plant-derived exosomal microRNAs inhibit lung inflammation induced by exosomes SARS-CoV-2 Nsp12. Mol Ther. 2021;29(8):2424–2440. doi:10.1016/j.ymthe.2021.05.005
166. Rabienezhad Ganji N, Urzì O, Tinnirello V, et al. Proof-of-concept study on the use of tangerine-derived nanovesicles as siRNA delivery vehicles toward colorectal cancer cell line SW480. Int J Mol Sci. 2023;25(1):546. doi:10.3390/ijms25010546
167. Zu M, Xie D, Canup BSB, et al. ‘green’ nanotherapeutics from tea leaves for orally targeted prevention and alleviation of colon diseases. Biomaterials. 2021;279:121178. doi:10.1016/j.biomaterials.2021.121178
168. Cao M, Diao N, Cai X, et al. Plant exosome nanovesicles (PENs): green delivery platforms. Mater Horizons. 2023;10(10):3879–3894. doi:10.1039/D3MH01030A
169. Rutter BD, Innes RW. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiology. 2017;173(1):728–741. doi:10.1104/pp.16.01253
170. Mitchell MJ, Billingsley MM, Haley RM, et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
171. Mu N, Li J, Zeng L, et al. Plant-derived exosome-like nanovesicles: current progress and prospects. Int J Nanomed. 2023;18:4987–5009. doi:10.2147/IJN.S420748
172. Mao X, Li T, Qi W, et al. Advances in the study of plant-derived extracellular vesicles in the skeletal muscle system. Pharmacol Res. 2024;204:107202. doi:10.1016/j.phrs.2024.107202
173. Liu R, Zhang F, He X, et al. Plant derived exosome-like nanoparticles and their therapeutic applications in glucolipid metabolism diseases. Journal of Agricultural and Food Chemistry. 2025;73(11):6385–6399. doi:10.1021/acs.jafc.4c12480
174. Wang B, Zhuang X, Deng ZB, et al. Targeted drug delivery to intestinal macrophages by bioactive nanovesicles released from grapefruit. Mol Ther. 2014;22(3):522–534. doi:10.1038/mt.2013.190
175. Guimarães D, Cavaco-Paulo A, Nogueira E. Design of liposomes as drug delivery system for therapeutic applications. Int J Pharm. 2021;601:120571. doi:10.1016/j.ijpharm.2021.120571
176. Wang C, Lan X, Zhu L, et al. Construction strategy of functionalized liposomes and multidimensional application. Small. 2024;20(25):e2309031. doi:10.1002/smll.202309031
177. Beach MA, Nayanathara U, Gao Y, et al. Polymeric nanoparticles for drug delivery. Chem Rev. 2024;124(9):5505–5616. doi:10.1021/acs.chemrev.3c00705
178. Yasamineh S, Yasamineh P, Ghafouri Kalajahi H, et al. A state-of-the-art review on the recent advances of niosomes as a targeted drug delivery system. Int J Pharm. 2022;624:121878. doi:10.1016/j.ijpharm.2022.121878
179. Xu M, Qi Y, Liu G, et al. Size-dependent In vivo transport of nanoparticles: implications for delivery, targeting, and clearance. ACS Nano. 2023;17(21):20825–20849. doi:10.1021/acsnano.3c05853
180. Ou X, Wang H, Tie H, et al. Novel plant-derived exosome-like nanovesicles from catharanthus roseus: preparation, characterization, and immunostimulatory effect via TNF-α/NF-κB/PU.1 axis. J Nanobiotechnol. 2023;21(1):160. doi:10.1186/s12951-023-01919-x
181. Sriwastva MK, Deng ZB, Wang B, et al. Exosome-like nanoparticles from mulberry bark prevent DSS-induced colitis via the AhR/COPS8 pathway. EMBO Rep. 2022;23(3):e53365. doi:10.15252/embr.202153365
182. Kim SQ, Kim KH. Emergence of edible plant-derived nanovesicles as functional food components and nanocarriers for therapeutics delivery: potentials in human health and disease. Cells. 2022;11(14):2232. doi:10.3390/cells11142232
183. Zhang L, He F, Gao L, et al. Engineering exosome-like nanovesicles derived from asparagus cochinchinensis can inhibit the proliferation of hepatocellular carcinoma cells with better safety profile. Int J Nanomed. 2021;16:1575–1586. doi:10.2147/IJN.S293067
184. Verheijden RJ, van Eijs MJM, May AM, et al. Immunosuppression for immune-related adverse events during checkpoint inhibition: an intricate balance. Npj Precision Oncology. 2023;7(1):41. doi:10.1038/s41698-023-00380-1
185. Caracciolo G. Artificial protein coronas: directing nanoparticles to targets. Trends Pharmacol Sci. 2024;45(7):602–613. doi:10.1016/j.tips.2024.05.003
186. Kalarikkal SP, Kumar MN, Rajendran S, et al. Natural plant-derived nanovesicles for effective psoriasis therapy via dual modulation of IL-17 and NRF2 pathway. Iscience. 2025;28(6):112556. doi:10.1016/j.isci.2025.112556
187. Tan S, Liu Z, Cong M, et al. Dandelion-derived vesicles-laden hydrogel dressings capable of neutralizing staphylococcus aureus exotoxins for the care of invasive wounds. J Control Release. 2024;368:355–371. doi:10.1016/j.jconrel.2024.02.045
188. Chen J, Wu J, Mu J, et al. An antioxidative sophora exosome-encapsulated hydrogel promotes spinal cord repair by regulating oxidative stress microenvironment. Nanomedicine. 2023;47:102625. doi:10.1016/j.nano.2022.102625
189. Wang Q, Ren Y, Mu J, et al. Grapefruit-derived nanovectors use an activated leukocyte trafficking pathway to deliver therapeutic agents to inflammatory tumor sites. Cancer Res. 2015;75(12):2520–2529. doi:10.1158/0008-5472.CAN-14-3095
190. Suk JS, Xu Q, Kim N, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Delivery Rev. 2016;99(Pt A):28–51. doi:10.1016/j.addr.2015.09.012
191. Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119(2):236–244. doi:10.1016/j.jconrel.2007.02.010
192. McSweeney MD, Wessler T, Price LSL, et al. A minimal physiologically based pharmacokinetic model that predicts anti-PEG IgG-mediated clearance of PEGylated drugs in human and mouse. J Control Release. 2018;284:171–178. doi:10.1016/j.jconrel.2018.06.002
193. Baytas SN, Linhardt RJ. Advances in the preparation and synthesis of heparin and related products. Drug Discovery Today. 2020;25(12):2095–2109. doi:10.1016/j.drudis.2020.09.011
194. Liam-Or R, Faruqu FN, Walters A, et al. Cellular uptake and in vivo distribution of mesenchymal-stem-cell-derived extracellular vesicles are protein Corona dependent. Nature Nanotechnol. 2024;19(6):846–855. doi:10.1038/s41565-023-01585-y
195. Yang LY, Liang GW, Cai BJ, et al. Enhanced tumor self-targeting of lemon-derived extracellular vesicles by embedding homotypic cancer cell membranes for efficient drug delivery. J Nanobiotechnol. 2025;23(1):74. doi:10.1186/s12951-025-03161-z