Advanced type of nanoparticle could help fight hard-to-treat cancers
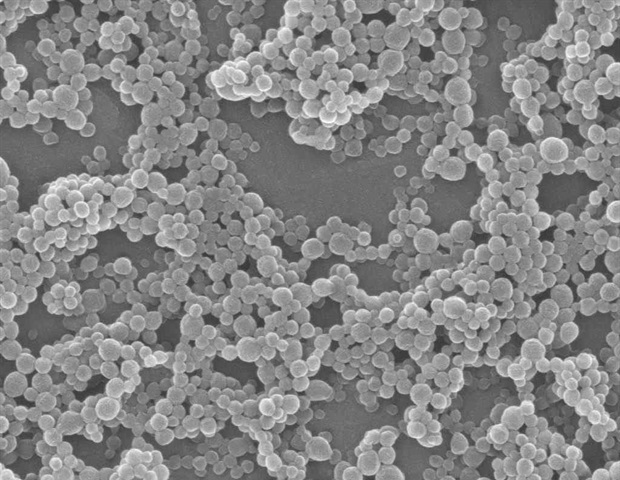

Nanoparticles, or tiny molecules that can deliver a payload of drug treatments and other agents, show great promise for treating cancers. Scientists can build them in various shapes with different materials, often as porous, crystal-like structures formed by a lattice of metal and organic compounds, or as capsules that enclose their contents inside a shell. When injected into a tumor, these particles can release treatments that attack cancer cells directly or complement other treatments like immunotherapy and radiation.
In a collaborative effort by cancer specialists and chemists, researchers at the University of Chicago have formulated an advanced type of nanoparticle that carries a compound derived from bacteria to target a potent immune system pathway called STING. The particles disrupt the tumor’s blood vessel structure and stimulate an immune response. This approach also helps overcome resistance to immunotherapy treatments in certain pancreatic tumors and boosts response to radiation therapy in glioma as well.
This was an unusual collaboration between medicine and inorganic chemistry to solve this unmet need of treating tumors that are intractable to conventional therapy. We were able to deliver an immune stimulant that has anti-tumor activity on its own, and enabled radiation and immunotherapy to cure these tumors.”
Ralph Weichselbaum, MD, the Daniel K. Ludwig Distinguished Service Professor and Chair of Radiation and Cellular Oncology at UChicago
The study, “Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration,” was published in Nature Nanotechnology on October 26, 2022.
Cold, hot, and hotter tumors
As always with cancer, some tumors prove resistant to even the most high-tech of treatments. Immunotherapy unleashes the body’s immune system to find and destroy cancer cells, but the tumors must be “hot” or inflamed for these treatments to be effective. So called “cold” tumors that aren’t inflamed can hide from the immune system but continue to grow and metastasize.
In a pair of studies published in 2014, Weichselbaum and other UChicago researchers showed that mice that lacked a protein pathway called STING did not mount an effective immune response to cancer in conjunction with immunotherapy or high-dose radiation treatment. STING, short for Stimulator of Interferon Genes complex, is a crucial part of the process the immune system relies on to detect threats–such as infections or cancer cells-;that are marked by the presence of DNA that is damaged or in the wrong place, inside the cell but outside the nucleus.
Since then, STING has become an enticing target for treatments to heat up cold tumors and make already hot tumors hotter. Doing so has been a challenge, however, because drugs that stimulate the STING pathway tend to be very small and water soluble, so when they are injected intravenously, they are cleared quickly by renal filtration and can cause toxicity to normal tissues at high doses.
Wenbin Lin, PhD, the James Franck Professor of Chemistry at UChicago, specializes in building nanostructures that can deliver a variety of compounds to tumors. Nanoparticles tend to get trapped in tumors because of their haywire vasculature and lymphatic systems, thus they can deliver more of their payloads exactly where needed. Lin has developed a new type of particle called nanoscale coordination polymers (NCPs) that have a non-toxic zinc phosphate core surrounded by layers of lipids. These NCPs have the advantage that they can be engineered for controlled release, further increasing drug deposition in tumors.
Lin, who is trained as an inorganic chemist, says he is in a unique situation working on medical treatments because of his experience designing particles with different properties. “It’s a unique technology that is well-suited for delivering many drug agents. We already know how to modify the surface so they can circulate in the blood and not be engulfed by macrophages,” he said.
A versatile technology
In the new study, Weichselbaum and Lin’s teams loaded the NCPs with a nucleotide called cyclic dimeric adenosine monophosphate (CDA). CDA is a bit of DNA that bacteria generate when they invade a host; its sudden appearance-;whether by infection or dropped off by a nanoparticle-;triggers the STING pathway and the host’s innate immune response to fight the cancer.
This boosted immune response attacked the tumors in multiple ways, suppressing tumor growth and preventing metastasis in several types of cancers. It disrupted endothelial cells in the blood vessels of tumors, further increasing the deposition of CDA in tumors. Surprisingly, it also enhanced the ability of tumor-associated macrophages that had infiltrated tumors to present antigens that mark them for attack by anti-tumor T-cells.
In addition, this approach made non-inflamed, cold pancreatic tumors more susceptible to immunotherapy treatment. It was also effective against glioma, effectively crossing the blood-brain barrier to reverse resistance to immunotherapy and enhance the effects of radiation treatments.
“That’s the brilliant part of these nanoformulations. We were able to encapsulate a STING agonist that is extremely potent and promotes both innate and adaptive immunity,” Weichselbaum said.
Lin, who has formed a startup company called Coordination Pharmaceuticals to develop NCPs, is enthusiastic about their potential for more clinical uses.
“This has tremendous potential because we’re not limited to a single compound. We can formulate other nucleotides and use other drugs in the same NCP,” he said. “The technology is versatile, and we are exploring ways to optimize formulations to take more NCP candidates into clinical trials. Small startups can advance clinical candidates in a much shorter amount of time than big drug companies.”
Yang, K., et al. (2022) Zinc cyclic di-AMP nanoparticles target and suppress tumours via endothelial STING activation and tumour-associated macrophage reinvigoration. Nature Nanotechnology. doi.org/10.1038/s41565-022-01225-x.



