Nanotechnology platform sensitizes cancer cells to immunotherapy

Immunotherapy – harnessing the body’s own immune system to fight cancer – has the potential to revolutionize cancer care. But while blood cancers such as leukaemia and lymphoma respond well to cancer immunotherapy, solid tumours display a limited response.
One possible reason for this disparity is the varied expression of surface proteins in different cancers. For example, the membrane-bound protein SLAMF7 – which activates the immune system and prompts phagocytosis (ingestion) of cancer cells by immune cells – is expressed by blood cancer cells, but not by solid tumours.
With the aim of making solid tumour cells more receptive to immunotherapy, researchers at The University of Texas MD Anderson Cancer Center have developed a nanotechnology platform that triggers their expression of SLAMF7. Described in Nature Nanotechnology, the platform is based on bispecific tumour-transforming nanoparticles (BiTNs) that comprise a polymeric core conjugated with tumour-targeting ligands and SLAMF7.
“With this new platform, we now have a strategy to convert a solid tumour, at least immunologically, to resemble a haematological tumour, which often has a much higher response rate to immunotherapy treatments,” says Wen Jiang, who co-led the study along with Betty Kim. “If we are able to translate and validate this approach in the clinic, it may enable us to get closer to the maximum level of activity from immunotherapy drugs with cancers that have not traditionally responded well.”
In vitro and in vivo assessment
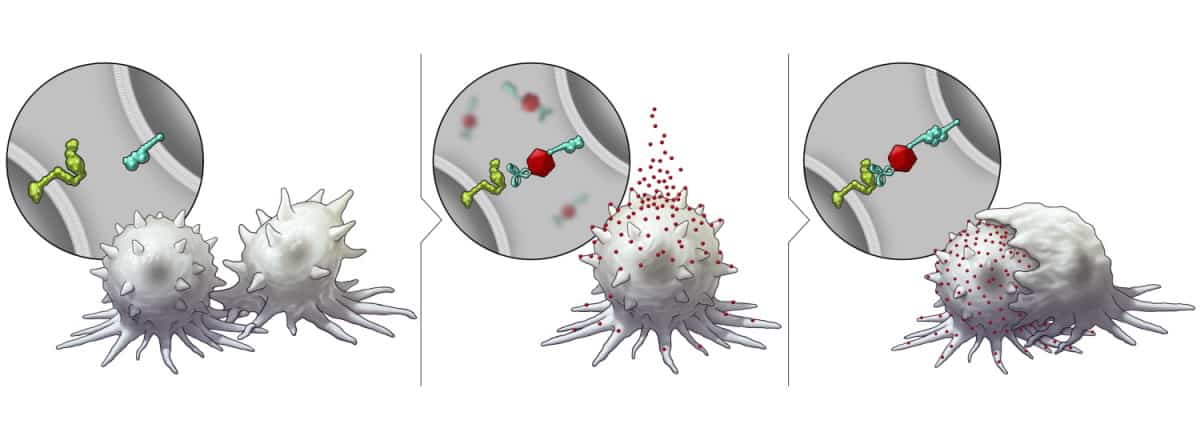
The researchers first investigated the platform in vitro, using HER2-positive breast cancer cells. They created cell-specific BiTNs for this application by conjugating the nanoparticles with anti-HER2 antibodies to bind to the tumour cells, and SLAMF7 to activate an immune response. The resulting nanoconjugate – BiTNHER – selectively targeted HER2-positive breast cancer cells and labelled them with SLAMF7.
The team confirmed that the BiTNHER-labelled cancer cells provoked a higher level of phagocytosis compared with unconjugated nanoparticles. BiTNHER also sensitized the breast cancer cells to treatment with an anti-CD47 antibody, which blocks the “don’t eat me” signal from tumour cells, further elevating phagocytic activity.
Next, the team evaluated BiTNHER in mice with breast cancer tumours of either TUBO cells, which express the rodent version of HER2, or 4T1 cells that lack this receptor. Treatment with BiTNHER plus anti-CD47 significantly reduced tumour burden and prolonged the survival of mice with TUBO tumours; the anti-tumour effect was not observed in the 4T1 tumours.
The researchers note that the combination treatment led to significant tumour inhibition compared with BiTNs or anti-CD47 alone. A long-term toxicity study found no significant difference in blood counts between untreated and treated mice.
To demonstrate the versatility of the BiTN platform, the researchers customized the nanoparticles to target another tumour receptor – the folate receptor expressed by triple-negative breast cancer. They created BiTNFo by replacing the anti-HER2 antibody with folate. BiTNFo targeted and transformed cancer cells into SLAMF7-expressing cells. As expected, incubating 4T1 cells with BiTNFo and anti-CD47 led to greater phagocytosis than seen for TUBO cells.
“Because these are engineered constructs, this can be used as a plug-and-play approach to incorporate different tumour-targeting agents or immune molecules onto the surface of the nanoparticle,” says Kim in a press statement.
The researchers also tested BiTNFo in a spontaneous 4T1 metastasis mouse model, treating the primary tumours with BiTNFo and anti-CD47 before surgical resection. This combination inhibited local disease recurrence but did not reduce distant metastases or prolong overall survival. Adding anti-PD1 to the treatment, however, led to prolonged metastasis inhibition, with two of seven mice showing long-term tumour-free survival.
Immunotherapy plus a burst of radiation treats brain tumours in mice
Finally, to further enhance the translational relevance of this model, the team investigated a post-surgery treatment regime. Here, tumours in mice were resected on day 12 without any pre-treatment, and then from day 15, the animals were treated with the triple combination of BiTNFo, anti-CD47 and anti-PD1. This post-operative treatment inhibited metastasis and prolonged survival – indicating that even without intratumoural treatment, BiTNs can help eliminate residual tumour cells and reduce systemic disease.
Next, the researchers are focusing on translating this new technology into the clinic. “To make clinical translation easier, we are exploring a protein-based strategy in which we will develop a bispecific protein that can act similarly as the BiTN,” Jiang tells Physics World. “This will require some protein/antibody engineering but will likely be easier to satisfy regulatory approvals.”