Advancement in Therapeutic Intervention of Prebiotic-Based Nanoparticles for Colonic Diseases
Introduction
Along with distinct changes in people’s lifestyle and diet structure, the incidence of colonic diseases (CDs) such as colitis and colon cancer is increasing year by year.1,2 Moreover, some CDs are not easy to be detected at first followed by a long course of illness and relapse,3,4 which lays a huge hidden risk for public health. At the same time, as the terminal tissue of the intestine, the sophisticated structure, function and internal environment of the colon with diverse bacteria bring great challenges to the diagnosis ex juvantibus of CDs.5,6
Currently, the pathogenesis and relevant mechanisms of most CDs have not been completely clear. In addition to a fraction of cases caused by genetic factors, environmental factors (eg, diet, lifestyle, psychophysiology, and oxidative stress) and pathogenic infection play a leading role in the occurrence and development of CDs.7 These factors lead to damage to the intestinal mucosa, disruption of the intestinal epithelial barrier, and dysbiosis of the intestinal flora, which resultantly alter the absorption profile of foods. The change in nutrient absorption in turn causes imbalances in the gut microbiota, further worsening the intestinal lesion. Indeed, there are more than 100 trillion viable microbes within the healthy human intestine.8 Our coexistence with the gut microbiota denotes a dynamic and mutually beneficial relationship, a major determinant of health and disease. In this regard, the significant role of the gut microbiota in the pathogenesis of CDs supports proof of concept that modification of gut microbiota can intervene CDs. Improving the intestinal flora is becoming a new breakthrough in the treatment of CDs.
Prebiotics, a class of non-digestive substances able to be harnessed by beneficial bacteria to improve the gut micro-ecosystem, are potential tools for regulating the gut microbiota.9 Prebiotics have attracted much attention for their pH sensitivity, gastric resistance, and colonic degradability by microbiota as well as modifiable nature. Furthermore, it has been shown that prebiotics can improve a variety of physicochemical properties of drugs, such as solubility, instability, and dispersibility.10 The merits provided with prebiotics enable them ideal materials for constructing colon-targeted drug delivery systems (CTDDs). Therefore, prebiotic-based CTDDs that integrate intestinal microbiota modulation and medication are expected to become a promising strategy for the treatment of CDs.
Considering the suitability of prebiotics for colon-specific drug delivery and CD intervention, this work contributes to dissect the applicable potentials of prebiotic nanoparticles (NPs) in the remedy of CDs. This review begins with the basic information of CDs and the gut microbiota followed by their interplay and regulating approaches. The species of prebiotics as well as their mechanisms acting on intestinal flora and the design rationale of prebiotic NPs for colon-specific drug delivery were generalized. Finally, the efficacy and suitability of prebiotic nanomedicines were appreciated with reported examples.
Pathophysiology of Colonic Diseases
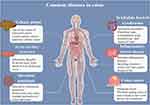
Under the influence of various internal and external detrimental factors, different pathological changes take place in the colon tissue, resulting in the occurrence of CDs. Common diseases of the colon include colonic polyps,11 acute bacillary dysentery,12 intestinal amebiasis,13 irritable bowel syndrome (IBS),14 inflammatory bowel disease (IBD),15 colon cancer,16 etc. (Figure 1). CDs also incorporate the colon melanosis, a rare, non-inflammatory, benign, and reversible disease usually associated with the persistent use of laxatives.17 If these CDs are not treated in time, they will cause intestinal dysfunction and malabsorption of nutrients, which not only aggravate the primary diseases, but even give rise to serious complications, such as toxic megacolon, colon stenosis or obstruction, major bleeding, and eventually evolving into malignancies. A large number of clinical cases show that long-term incurable colitis is a predisposing condition for colorectal cancer.18 This has become one of the third most prevalent causes of cancer in the world.
 |
Figure 1 Common colonic diseases and their characteristics. |
Although the etiology and pathogenesis of most CDs are not yet fully understood, cumulative evidence underlines the involvement of both environmental and genetic factors that lead to the disintegration of intestinal epithelial barrier, mucosal damage and immune dysregulation response to the intestinal flora, resulting in the occurrence of colonic lesions.19 Among these, environmental factors, including but not limited to psychosocial aspect, dietary structure, lifestyle, long-term chronic inflammatory stimuli, and pathogenic microbial infection, dominate the colonic lesion. In addition, it demonstrated the importance of intestinal microbiota in maintaining homeostasis in the human digestive tract.20 It has also been pointed out that the intestinal microbiota is involved in the pathogenesis of CDs that is further associated with diet, lifestyle and nutrition intake. Besides correlation with the colonic lesion, the gut microbiota is implicated in promoting the release of inflammatory factors and colorectal carcinogenesis via multiple molecular mechanisms.21–23 Therefore, the treatment of CDs must highlight a profound understanding of the pathogenesis and a combination treatment with an applicative medication.
Significance of Intestinal Flora and Remedy Thereof
Intestinal flora, also known as intestinal or gut microbiota, refer to thousands of microorganisms in the human gastrointestinal tract (GIT), which are combined in a certain proportion, mutually restrictive and interdependent manner, to form a large and complex micro-ecosystem. The impact of the gut microbiota on health is profound. Abnormal gut microbiota can alter the levels of inflammatory cells, mediators, and neurotransmitters, which in turn cause lesions such as neuroinflammation.24 The colonic microbiome is the largest microbial community in our body. The micro-ecological environment may be a trigger for genetic and epigenetic changes in the colonic somatic cells, so intestinal flora cannot be ignored in the study of CDs. Numerous studies have shown that the richness and diversity of intestinal flora are linked to CDs and that the interaction between gut microbes and the host is complex and bidirectional.25,26 On the one hand, the imbalance of intestinal flora in the ecological system is considered to be a major factor in promoting the initiation and development of colonic lesions. Imbalance of intestinal flora can lead to colonic lesions through the following mechanisms: (1) affecting pathogenic microorganisms and their products; (2) changing metabolites in the circulation to cause colonic lesions; (3) destroying the host’s autoimmune surveillance; and (4) inducing inflammation and immunosuppression.27,28 In addition, gut microbiota dysbiosis is also implicated to have potential effects on the efficacy of different therapeutic strategies, including surgery, chemotherapy, radiotherapy, and immunotherapy.29 Furthermore, there is a crosstalk between colonic lesion and gut microbiota. Colonic lesions affect the species and function of gut microbiota, resulting in a decrease in beneficial bacteria as well as their diversity and excessive increase in pathogenic bacteria. Therefore, gut microbiota can be used as an important marker for the diagnosis and prognosis of CDs.
There is widespread interest in intervening some diseases by regulating the gut microbia. Approaches aiming to reverse gut microbial disorders include dietary intervention, fecal microbiota transplantation (FMT), probiotic or prebiotic supplementation, etc.30 Microbial nutriology has shown that the composition of gut microbiota is largely related to the host diet and the dietary structure. A long-term adherence of changing dietary habits and patterns can cause alteration in gut microbiota of the host.31,32 However, this approach is short-lived, and once the original dietary structure is restored, the intestinal flora will change accordingly. FMT denotes a prompt means of treatment by directly altering the composition of gut microbiota. It refers to the acquisition of intestinal microorganisms from the feces of healthy donors and transplantation into the patient’s intestinal tract whereby to change the composition of the patient’s intestinal flora, regulating the imbalance of gut microbiota and rebuilding the intestinal micro-ecosystem.33 FMT has been used to treat a variety of microbiome-related diseases, such as chronic constipation, IBS, IBD, metabolic syndrome, and autoimmune diseases.34 Rigorous screening of healthy donors, standardized operation and individualized clinical workflows are prerequisites for ensuring the quality of the fecal microorganisms acquired, the efficacy of FMT treatment, and the reduction of potential transplantation-related risks. Besides dietary management and FMT, supplementation with probiotics or prebiotics has also become an important means of regulating the intestinal flora.35 Probiotics are a combination of live beneficial bacteria and/or yeasts that naturally live in our body, such as bifidobacteria and lactobacilli. Probiotics can form the dominant intestinal flora to balance and improve gut microbiota by temporarily colonizing and producing metabolites to inhibit the growth of harmful bacteria while forming a natural barrier by adhering to the GI tract surface, thereby maintaining the homeostasis of the GI environment. There have been many attempts of applying probiotics to regulate the intestinal flora for treating Crohn’s disease, ulcerative colitis (UC) and IBS.36 Nevertheless, it is not a permanent approach to regulate the species of gut microbiota by supplementing probiotics due to several shortcomings of exogenous probiotics such as easy inactivation in the digestive tract, poor colonization ability, and short residence time.37 To this end, enhancive focus is being placed on supplementation of prebiotics. It turns out that the use of prebiotics is one of the effective approaches to regulate the gut microbiota.38 Compared with probiotics, prebiotics are indigestible food constituents that alter the species of the microbiome, hence the health of the host, by selectively stimulating the metabolism and proliferation of one or more beneficial bacteria present in the gut. For example, oligofructose and inulin can stimulate the growth of bifidobacteria and thus alter the balance of the intestinal flora, resulting in a healthier colonic micro-ecology.39
Prebiotics-Definition, Type and Sources
In 2016, the International Scientific Association for Probiotics and Prebiotics (ISAPP) redefines prebiotics as “a substrate that is selectively utilized by host microorganisms conferring a health benefit”.40 In the past 20 years, with the update of the definition of prebiotics and continuous discovery of other prebiotic active substances, the classification and characteristics of prebiotics have been gradually systematized. The earliest identified prebiotics are some oligosaccharides difficult to be digested, like oligofructose. Next, more and more oligo- and polysaccharides have been reported to have beneficial effects on gut microbiota. Among these, inulin and oligofructose represent the most studied and commercially successful functional prebiotics so far.41 Currently, besides some indigestible oligosaccharides such as galactooligosaccharide, xylooligosaccharide, and soybean oligosaccharide, some polysaccharides (eg, ganoderma polysaccharides and carrot nitrogenous polysaccharides), plant and herbal extracts (eg, anthocyanins and quercetin), protein hydrolysates (eg, α-whey protein and lactoferrin), and polyols (eg, xylitol, mannitol, sorbitol, and lactitol) have also demonstrated the prebiotic effects. In general, prebiotics contain both carbohydrates and non-carbohydrates.
There are two dominating types of carbohydrate prebiotics: functional oligosaccharides and polysaccharides. Oligosaccharides are non-digestible oligomeric saccharides generally consisting of 2–10 monosaccharide units linked by glycosidic bonds. Due to the lack of digestive enzymes that can break the bonds between these oligosaccharides, these functional oligosaccharides can pass through the upper digestive tract intactly while be degraded into short-chain fatty acids by special hydrolases secreted by microorganisms in the colon, thus regulating intestinal flora to give rise to beneficial effects on our body.42 Depending on their beneficial effects on gut microbiota, functional oligosaccharides have been used to prevent and treat a variety of diseases, including digestive, immune, metabolic, cancerous and neuropsychiatric disorders.43 Polysaccharides have a longer sugar chain consisting of at least 10 monosaccharides. Among them, inulin, β-glucan, pectin, seaweed polysaccharides, resistant starch and dextrin are the polysaccharides that have been proved to have prebiotic effects. Inulin is a fructan with a higher degree of polymerization than oligofructose. Dietary innulin has been shown to improve the composition of gut microbiota in several clinical trials.44 Compared with inulin, β-glucan can promote the growth of bifidobacteria better and further regulate the gut immunity and relieve inflammation, showing the greater potential of prebiotics.45 Pectin is a complex anionic polysaccharide derived from plants, while seaweed polysaccharide is extracted from algae or microalgae. Pectin, seaweed polysaccharide and their derivatives are considered as good candidates of prebiotics in terms of preclinical studies. Many studies prove that resistant starch and dextrin, a specific hydrolysate of starch, can regulate the number and composition of intestinal flora and promote the growth of intestinal beneficial bacteria.46,47 In addition, dietary polysaccharides, as an important prebiotic resource, have been reported to alleviate colitis by regulating the composition of the gut microbiota.48 Common functional oligosaccharides and polysaccharides with prebiotic effects are summarized in Table 1.
 |
Table 1 Typical Prebiotics of Carbohydrates |
Non-carbohydrate prebiotics are some fatty acids, naturally occurring polyphenols, and botanical compositions. Currently, conjugated linoleic acid (CLA) and polyunsaturated fatty acids (PUFA) are identified as prebiotics, and their effect on the gut microbiota has been experimentally confirmed.64,65 It has been shown that CLA and PUFA can reversibly enhance the abundance of several genera or phylum such as Firmicutes, Bifidobacterium, Lactobacillus and Bacteroidetes in healthy human body, and increase the diversity of intestinal flora. In addition, the interplay between natural phytochemicals (eg, phenolic acids, flavonoids, stilbenes, tannins, and lignans, among others) and intestinal flora is arousing considerable interest for the study of prebiotics. The prebiotic effect of polyphenols is mainly attributed to the fact that polyphenol compounds and their metabolites that are not absorbed by the small intestine can regulate the ecological balance of intestinal microorganisms after reaching the colon, thus exerting a beneficial effect on health. For example, quercetin, anthocyanins, taxifolin, red wine polyphenols, blueberry polyphenols, dietary phenolic compounds (eg, garlic polysaccharides and maltopolysaccharides), carotenoids, and polymethoxylated flavones have exhibited certain regulatory effects on the intestinal flora and can be used as candidates of prebiotics.66–69
Regulatory Effects and Mechanisms of Prebiotics on CDs
Prebiotics can directly or indirectly modulate the species and structure of intestinal flora and transform the gut microbiota to the side beneficial to the health of the host. The underlying mechanisms of prebiotics intervening CDs relate to improving the activity of related enzymes to regulate their metabolic function, inducing local or systemic immune responses beneficial to the host health, promoting the intestinal epithelial regeneration, increasing the secretion of mucin, providing antioxidant effect, protecting the intestinal epithelial tight junctions, etc., as illustrated in Figure 2.
 |
Figure 2 Regulatory activities of prebiotics on CDs: (A) direct effects and (B) indirect effects on the intestinal function. |
The direct effects of prebiotics on CDs are manifested by their positive modulation on the intestinal flora, enterocytes, and immune cells. Prebiotics, as a source of energy for the host, can be directly utilized by the intestinal beneficial bacteria to promote their growth and reproduction, thus forming micro-ecological competitive advantages. Also, they can increase the absorption of certain nutrients, adsorb intestinal pathogens in the digestive tract and promote their excretion with feces, and improve the intestinal micromilieu in addition to a laxative effect. At the same time, it can inhibit the growth of harmful bacteria by directly inhibiting the expression of harmful genes. When ingested, prebiotics enter the intestine and come into direct contact with intestinal epithelial cells. Studies have shown that prebiotics are toll-like receptor 4 (TLR4) ligands in the intestinal epithelial cells (IECs), which induce the production of a series of anti-inflammatory cytokines such as interleukin 10 (IL-10) and transforming growth factor-β (Tgfβ) and inhibit inflammatory cytokines whereby to alleviate the intestinal inflammation.70 Prebiotics can also ameliorate the integrity of the physiological barrier by inducing specific tight junction proteins through the protein kinase C-δ (PKCδ)-dependent pathway to prevent pathogen-induced barrier disruption.71 Moreover, prebiotics can upregulate the expression of galectin-9, reduce acute allergic skin reactions and mastocyte degranulation, and promote Th1 and Treg production to prevent allergic symptoms.72 The third effect of prebiotics on CDs is to directly affect immune cells in the gut. Prebiotics can both induce the secretion of anti-inflammatory (IL-10) and pro-inflammatory cytokines (IL-1β and TNF-α) via blood monocytes through activating the NF-kB pathway by binding to TLR4. They can also bind pathogen-recognition receptors (PRRs) on the surface of dendritic cells to induce the secretion of IL-10. Furthermore, prebiotics enhance the secretion of IL-10 and interferon-γ (IFN-γ) by CD4+ T lymphocytes.73
The indirect effects of prebiotics on the intestinal function mainly depend on their ability to promote the production of short-chain fatty acids (SCFAs), of which acetic acid, propionic acid, and butyric acid are the most important SCFAs.74 SCFAs are metabolites of prebiotics fermented by the intestinal flora, which can be utilized by the gut microbiota for its own metabolism or released into the lumen, playing a beneficial role in many ways. For example, SCFAs can lower the pH of the lumen and inhibit the growth of exogenous pathogenic bacteria and intestinal spoilage organisms, thus reducing the production of toxic substances; SCFAs can specifically interact with IECs or immune cells to alter various cellular metabolic processes as well as gene expression, differentiation, proliferation, and apoptosis.75 One mechanism is that SCFAs act as a link between the microbiota and the immune system by modifying several cellular processes of IECs and leukocytes development, survival and function through activating G protein-coupled receptors (eg, FFAR2, FFAR3, GPR109a and Olfr78) and modulating the activity of enzymes and transcription factors. SCFAs are also able to enter the cytoplasm via transporters (eg, Slc16a1 and Slc5a8) or passive diffusion across the plasma membrane (mainly in the non-ionized form), where they modulate the activity of several enzymes and transcription factors, including HIF, HDACs and HAT.76 The antibacterial and metabolic regulatory effects of SCFAs as well as their role in central appetite regulation have also been well elucidated.77–79 In addition, SCFAs in the blood can interact with different immune cell subtypes and regulate immune signal transduction through a variety of ways to regulate the immune response.80
Fundamentals of Prebiotic NPs Design for CDs
Colon-targeted drug delivery systems (CTDDs) are designed for specific delivery of a therapeutic agent to the colonic site whereby to enhance the local drug exposure and hence the curative efficacy. Prebiotics can be used to construct single prebiotics-based NPs, to develop nanocomposites with oppositely charged polymers, and to decorate other NPs in order for prebiotic functionality (Figure 3). Depending on the feature of biomaterials used, prebiotic CTDDs (p-CTDDs) can be subcategorized into diverse systems further based on different drug release mechanisms. In general, there are four types of p-CTDDs, ie, single p-CTDDs, bioadhesive p-CTDDs, pH-sensitive p-CTDDs, redox-sensitive p-CTDDs, and multivariable p-CTDDs.
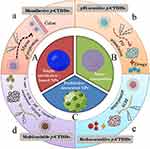 |
Figure 3 Prebiotics available for construction of single prebiotic NPs (A), nanocomposites (B) and prebiotics-decorated NPs (C) based on different drug release mechanisms (a–d). |
Single p-CTDDs
The realization of single p-CTDDs depends on the enzymatic metabolic process of intestinal flora and the prebiotic materials used. Prebiotics can be decomposed by enzymes (eg, azo reductase, esterase, pectinase, glucanase, etc.) produced by anaerobic bacteria present in the colon. The stomach and small intestine lack the corresponding enzymes, so single p-CTDDs are not digested and degraded in the upper digestive tract, and the drug will be securely delivered to the colon and slowly released there due to degradation of prebiotics by specific enzymes.81 In recent years, great interest is emerging for discovering suitable prebiotics to design single p-CTDDs. Ohno et al82 prepared glucan NPs for colonic delivery of curcumin and found that the nano-system could inhibit the development of dextran sulfate sodium (DSS)-induced colitis by inhibiting the activation of NF-κB in the colonic epithelial cells, inducing the expansion of Tregs in the colonic mucosa, and regulating the structure of intestinal microbiota. It is worth noting that the diversity of gut microbiota changes significantly in the disease state, which can affect the type and diversity of enzymes.83 Therefore, azo polymers and colonic enzyme-responsive materials cannot guarantee site-specific degradation of polymers, while prebiotics can overcome these shortcomings due to their unique physicochemical properties.
Bioadhesive p-CTDDs
The implementation of bioadhesive p-CTDDs leans upon the interaction between the bioadhesive materials and mucus glycoprotein to enable the drug adhere to the mucosal surface of the colon, so as to improve the local concentration and prolong the retention time of drug in the colon. For example, the presence of abundant carboxyl groups in sodium alginate makes the polysaccharide prebiotics be charged negatively and able to adhere to the mucus layer, which is conducive to its enrichment in the inflammatory site. Chiu et al84 prepared wheat germ agglutinin (WGA)-conjugated disulfide cross-linked sodium alginate NPs as a docetaxel carrier for colon cancer therapy. The colonic mucosa adhesion was significantly improved due to cross-linking with WGA, and the bioadhesive NPs also exhibited excellent responsiveness to pH and intracellular reducing environment.
pH-Sensitive p-CTDDs
pH-sensitive p-CTDDs are devised based on the difference in pH and microbiota composition in different parts of the digestive tract. It is a kind of formulation technology that combines pH-sensitive and microbiota-dependent principles to produce the colonic targeting effect. Carrier materials of such p-CTDDs are generally composed of prebiotics and pH-sensitive adjuvant (eg, Eudragit FS30D and Eudragit RL30D) or modified prebiotic derivatives with pH-responsive feature. More accurate colonic targeting can be achieved through this combined strategy. Quercetin (QUE), a natural flavonoid dietary antioxidant, has been shown to mitigate the experimental colitis in mice by modulating the function of colonic macrophages via a heme oxygenase-1-dependent pathway.85 Caddeo et al86 employed chitosan and xanthan gum to prepare QUE-loaded microparticles and coated them with Eudragit L100. The results showed that QUE was slowly released through non-Fickian diffusion in the colon under an alkaline environment. This approach is expected to prepare pH-sensitive microbiota-dependent p-CTDDs to deliver drugs that are poorly absorbed. Besides, pH-sensitive p-CTDDs can also be engineered by multilayer coating.
Redox-Sensitive p-CTDDs
Redox-sensitive p-CTDDs take advantage of the high redox environment of the disease site and the degradability of the delivery system by microbial enzymes. Under the normal physiology, the in vivo reactive oxygen species (ROS) keeps a dynamic equilibrium, but the hyperactive immune cells or cancer cells in the lesion site of the colon will produce a high concentration of ROS to break the balance.87 Furthermore, the high concentration of ROS will further damage the normal cells and aggravate the disease. ROS-sensitive drug delivery systems based on prebiotics can have dual effects of controlled release and removal of excessive ROS in inflammatory or tumorous tissue. Sun et al88 developed prebiotic NPs loading budesonide using 4-aminothiophene-carboxymethyl inulin (an amphiphilic inulin derivative) as the carrier material. In vivo/vitro experiments indicated that the constructed NPs had obvious redox sensitivity and tended to accumulate in the inflammatory site, showing a good colonic targeting.
Multivariable p-CTDDs
Combining multiple mechanisms such as pH, time, bioadhesion and microbial degradation, a new system of multivariable p-CTDDs arises. This type of p-CTDDs contains prebiotics and pH, time and/or other sensitive materials that are integrated in a physical or chemical way as vehicles to deliver therapeuticals to the colonic site. For instance, Nunthanid et al89 utilized 5-aminosalicylic acid as a model drug to develop time-, pH-, and enzyme-controlled multivariable p-CTDDs by spray-drying with chitosan acetate (CSA) and hydroxypropyl methylcellulose (HPMC). The multivariable p-CTDDs were designed based on the properties of swelling of CSA and HPMC in the gastric juice and lower solubility of CSA in the intestine. CSA just can be degraded in the colon by enzymes resulting from bacteria. The degradation of CSA is accelerated after reaching the colon through a time-delay effect of gel-like HPMC that produces a colon-specific effect on drug delivery.
Nanomedicines Based on p-CTDDs for CD Intervention
The emerging colonic drug delivery systems based on prebiotics include microspheres/capsules, micelles, nanoparticles, polyelectrolyte nanocomposites, etc. Among these, prebiotic NPs and nanocomposites have been proverbially investigated owing to the advantages of adjustability of size and shape, modifiability of surface function, and controllability of drug release. As far as colon-specific delivery is concerned, prebiotic NPs are also provided with excellent intestinal mucosal permeability and high uptake by macrophages, M cells and other intestinal cells in the modality of NPs, thus potentiating the curative effect and enhancing the colonic bioavailability of the payload.
NPs fabricated from prebiotics are intended to strengthen the physicochemical properties of therapeuticals or achieve preferable targeted drug delivery. Prebiotic NPs able to be precisely activated by the physiological environment of the colon promise great operability for colon-targeted drug delivery. Considerable efforts have been directed towards developing novel prebiotic-based nanovehicles, such as self-assembled prebiotic NPs, prebiotic-modified NPs, and prebiotic NPs-laden gels.90 Among various prebiotics, starch, alginate, chitosan, pectin, guar gum, hyaluronic acid and xanthan gum have been broadly explored for oral colon-specific drug delivery. The indigestibility in the upper GI tract and health benefits of prebiotics can be utilized to achieve colon-specific drug delivery and promote the proliferation of colonic probiotics. An increasing number of prebiotics and their derivatives are being developed as drug delivery vehicles. Interests mostly focus on the modification of prebiotics by substituting functional groups and the compatibility of different prebiotics to optimize their properties.91 Combining prebiotics with probiotics or probiotic spores to pursue a multiplicative effect is also a new attempt to maximize the therapeutic efficacy of CDs.92 Electrospinning with prebiotics as carrier materials that results in a colon-specific release of active substances can also effectively enhance a drug’s bioavailability and its curative effect.93 Prebiotic-coated vehicles have already been engineered as a therapeutic tool for the intervention of intestinal inflammatory diseases.94 Table 2 collects the representative p-CTDDs specific for CDs based on different prebiotic materials.
 |
Table 2 Representative Examples of p-CTDDs Applied in Treatment of Various CDs Based on Non-Clinical Investigation |
Prebiotics-based NPs minimize drug exposure to the upper GI tract due to their non-digestive property that enables drug to concentrate toward the colonic area, thus improving drug’s colonic bioaccessibility and reducing the side effects. Certain prebiotics such as polysaccharides can not only target drug to the colon through an enzyme-responsive mechanism but also cooperate with the payload to alleviate CDs owing to their good bioactivity and intestinal immunomodulatory actions. Simple prebiotic NPs without any active ingredient have shown good gut microbial and immunomodulatory effects. For instance, Chandrarathna et al108 developed Spirulina maxima-derived modified pectin (SmP) and NPs (SmPNPs) and evaluated their modulating effects on gut microbiota, immune responses, and gut morphometry. It was found that both modified SmP and SmPNPs showed excellent potential to modulate gut microbial community, enhance the expression of immune related genes, and improve gut morphology. In practice, prebiotics have served as adjuvant therapy for CDs. Phellinus igniarius polysaccharide (PIP) with anti-inflammatory and prebiotic activities was formulated into chitosan-modified PLGA NPs.109 The choreographed nanomedicine exhibited high phagocytosis by macrophages, inhibited M1-like macrophage phenotype, and regulated the inflammatory cytokines in vitro. It was revealed in vivo that the nanomedicine prevented intestinal inflammatory damage and protected the integrity of the intestinal barrier while increasing the content of SCFAs and positively regulating the gut microbiota. The enteropathogenic microorganisms were properly suppressed, but the beneficial microbiota were enriched, including Lactobacillus and Akkermansia. Modification of NPs with prebiotics also leads to favorable colonic drug delivery and therapeutic outcomes. For example, chondroitin sulfate-conjugated polymeric NPs (CS-NPs) were used to encapsulate curcumin for colonic macrophage-targeted drug delivery.110 Oral administration of chitosan/alginate hydrogel loading CS-NPs ameliorated the therapeutic index against UC in a murine model compared with conventional Cur-NPs. In another study, a programmed colonic drug release system based on polyacrylic acid, chitosan, and hydroxyapatite was developed for the integration of treatment and MRI imaging.111 The nano-system could protect, transport, and program drug release within the colonic environment and resulted in a significant therapeutic optimization. In addition, prebiotic materials have also been used to construct dual-targeted NPs to achieve a colonic drug delivery. Hou et al112 designed colonic dual-targeted NPs using polylactic acid-polyethyleneimine (PLA-PEI) and hyaluronic acid-inulin (HA-IN) for oral delivery of paclitaxel. Inulin (IN) that can resist degradation in the upper digestive tract as outer shell ensures the safe delivery of NPs to the colon while serving as an enzyme-sensitive component to release the anticancer agent within the colon under the action of colon-associated bacteria. The exposed HA not only promotes intestinal mucosal penetration of NPs but also undertakes the target of CD44 to play an active targeting role in tumor transport, as illustrated in Figure 4. Moreover, the in vivo studies disclosed that the resultant p-CTDDs could remain in vivo for 24 h and accumulate toward the tumor site. A satisfactory therapeutic outcome on the orthotopic colon cancer model was finally achieved. These eminent studies have laid the foundation for the healing of various CDs.
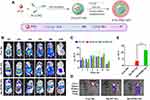 |
Figure 4 Colonic dual-targeted p-CDDs constructed by PLA-PEI and HA-IN (A). The colonic targetability was in vivo evaluated by biodistribution after oral administration of Dir, Dir/PP NPs and Dir/PPHI NPs in the orthotopic colon cancer model (B) based on bioluminescent quantitation (C), fluorescence imaging on the tumor and organ (D), and the relative bioluminescence on tumor after administration (E). **P < 0.01, unpaired two-tailed t-test. Notes: Reproduced from Hou Y, Jin J, Duan H, et al. Targeted therapeutic effects of oral inulin-modified double-layered nanoparticles containing chemotherapeutics on orthotopic colon cancer. Biomaterials. 2022;283:121440. © 2022 Elsevier Ltd. All rights reserved.112 |
Effectiveness and Safety of p-CTDDs
The use of prebiotic materials to construct p-CTDDs can not only result in targeted and controlled drug release in the colonic site but also achieve a synergistic effect with the payload by regulating the intestinal flora by virtue of their health benefits and peculiar properties. However, the efficacy and safety of p-CTDDs are important concerns for clinical applications. The effectiveness of p-CTDDs depends on impartial in vitro/vivo evaluation of p-CTDDs. Due to diversity in drug release modes of p-CTDDs prepared based on different principles, the in vitro/vivo characterization should be conducted with different techniques to confirm the colonic specificity and the release profile of the payload. Microbiota-dependent and multivariable p-CTDDs can be examined using a simulated colonic fluid by adding enzymes (eg, β-glycosidase and pectinase), cecal contents of rodents or human excreta, and/or colonic bacteria into a biorelevant medium.113 Tests were then performed selectively with buffers of different pH or at different intervals to mimic the in vitro release of drug upon undergoing different pH and transport time in the GI tract. Bayat et al114 established a rat inverted colon model using isolated ascending colon as an auxiliary tool for assessing the colon targeting, which is a relatively mature evaluation method in vitro. It has been reported that the use of γ-scintigraphy, usually using 99mTc to locate the stomach and intestine and other radioactive elements such as111In, 153Sm, and 171Er to label formulation, can monitor the transport process of CTDDs in vivo and determine the retention time in the stomach and the time of transporting through the small intestine and colon.115 In addition, tissue distribution analysis is also adopted as an in vivo tool to evaluate CTDDs. In this approach, animals are sacrificed at different times after administration, and colonic tissue homogenates are taken for drug quantification.116 A large number of studies have shown that p-CTDDS featured by both biomaterial and bioactivity can significantly improve the gastrointestinal stability of CTDDs and assure the reliability of colon-targeted drug release.91
Prebiotics have attracted increasing attention in the field of drug delivery due to their peculiar properties, such as rich sources, low cost, non-toxicity, good biocompatibility, and pleiotropic bioactivities. At present, there are no relevant reports on the toxic and side effects of prebiotics, and they are considered relatively safe as carrier materials. Currently used p-CTDDs are less toxic, which may be ascribed to the lower oral dose. Nevertheless, their potential toxicity to the body still remains alert. Firstly, the smaller particle size and higher specific surface area of nanomedicines indicate that some NPs can adsorb globulins, digestive enzymes and metabolic enzymes in GIT, which compromises the catalytic activity of enzymes and changes the normal function of GIT, thus reducing the digestion rate of starches, lipids or proteins. Secondly, NPs can penetrate the mucus layer and then be absorbed via an active or passive mechanism. After they are absorbed into cells, they can be metabolized, translocated to the blood, or accumulated in the cell, depending on the composition, particle size, aggregation state, and surface charge. When the accumulation of NPs in the cell exceeds a certain threshold, they can cause changes in the intracellular micromilieu that result in potential cytotoxicity. Moreover, indigestible NPs may interact with intestinal microbiota and change the nature of colonic microbiota, producing certain adverse effects on the body.117 Meanwhile, a long-term use of absorption promoter and surfactant that are co-formulated in p-CTDDs may give rise to intestinal epithelial injury and increase the risk of pathogens and toxins invading the intestinal lumen.118 Therefore, the safety of p-CTDDs becomes a concern for potential clinical applications.
Future Prospects
Despite many merits of prebiotic nanoparticles for CD intervention, there are still bottlenecks to develop prebiotic-based nanomedicines. Most prebiotic materials have no amphiphilicity and can hardly be used directly to encapsulate therapeutic molecules. Routinely, chemical modification is required to optimize the physicochemical properties of prebiotics. Also, prebiotic nanomedicines are greatly challenged by clinical translation due to the difficulty in quality control and high costs of clinical trials. It requires that prebiotic NPs can be manufactured continuously with good batch reproducibility and stability. Prebiotics are substances that have proven health benefits, which can benefit for a variety of CDs, especially those with intestinal flora dysbiosis. The integration of prebiotics and therapeutic molecule into nanomedicine will go a long way towards intervention of CDs. Next efforts should focus on fabricating novel functional prebiotics and advance the relevant clinical trials.
Conclusions
The colon has a complex and diverse structure, physiology and internal homeostasis, which confer a complicacy to medical intervention for CDs. Considering the importance of intestinal flora in the pathophysiology of CDs, it is of great interest to develop novel drug delivery systems with the gut microbiota as the therapeutic target. The use of prebiotics that can regulate the structure and function of intestinal flora to develop colon-targeting nanomedicines has become a research hotspot in recent years. Formulators have engineered a series of CTDDs using prebiotics based on various mechanisms, such as the microbiota-dependent enzymatic degradation in combination with in vivo redox environment, pH and time-response, intestinal mucosal adhesion, and receptor-molecular targeting. These p-CTDDs effectively deliver drug to the colonic site via the oral route with great success, significantly optimize the colonic bioavailability and curative effect, and reduce adverse reactions of drugs. Therefore, prebiotic-based NPs with a synergistic effect between payload and vehicle may provide a viable solution to the treatment of CDs.
Acknowledgment
This work was jointly supported by the Basic and Applied Basic Research Project of Guangzhou Science and Technology Plan (202201010743) and the Fundamental Research Funds for the Central Universities (No. 21622414).
Disclosure
The authors report no conflict of interest.
References
1. Du L, Ha C. Epidemiology and pathogenesis of ulcerative colitis. Gastroenterol Clin North Am. 2020;49(4):643–654. doi:10.1016/j.gtc.2020.07.005
2. Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020;158(2):341–353. doi:10.1053/j.gastro.2019.07.055
3. Ge C, Lu Y, Shen H, et al. Monitoring of intestinal inflammation and prediction of recurrence in ulcerative colitis. Scand J Gastroenterol. 2022;57(5):513–524. doi:10.1080/00365521.2021.2022193
4. Goéré D, Sourrouille I, Gelli M, et al. Peritoneal metastases from colorectal cancer: treatment principles and perspectives. Surg Oncol Clin N Am. 2018;27(3):563–583. doi:10.1016/j.soc.2018.02.011
5. Mosli M, Al Beshir M, Al-Judaibi B, et al. Advances in the diagnosis and management of inflammatory bowel disease: challenges and uncertainties. Saudi J Gastroenterol. 2014;20(2):81–101. doi:10.4103/1319-3767.129473
6. Adriani A, Ribaldone DG, Astegiano M, et al. Irritable bowel syndrome: the clinical approach. Panminerva Med. 2018;60(4):213–222. doi:10.23736/s0031-0808.18.03541-3
7. Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20(7):1082–1096. doi:10.2174/13816128113199990416
8. Koboziev I, Reinoso Webb C, Furr KL, et al. Role of the enteric microbiota in intestinal homeostasis and inflammation. Free Radic Biol Med. 2014;68:122–133. doi:10.1016/j.freeradbiomed.2013.11.008
9. Davani-Davari D, Negahdaripour M, Karimzadeh I, et al. Prebiotics: definition, types, sources, mechanisms, and clinical applications. Foods. 2019;8(3):92. doi:10.3390/foods8030092
10. Durazzo A, Nazhand A, Lucarini M, et al. An updated overview on nanonutraceuticals: focus on nanoprebiotics and nanoprobiotics. Int J Mol Sci. 2020;21(7):2285. doi:10.3390/ijms21072285
11. Pai RK, Bettington M, Srivastava A, et al. An update on the morphology and molecular pathology of serrated colorectal polyps and associated carcinomas. Mod Pathol. 2019;32(10):1390–1415. doi:10.1038/s41379-019-0280-2
12. Li S, Schmidt AM, Elliott SJ. Socioeconomic factors and bacillary dysentery risk in Jiangsu Province, China: a spatial investigation using Bayesian hierarchical models. Int J Environ Health Res. 2022;32(1):220–231. doi:10.1080/09603123.2020.1746745
13. Ishizawa R, Mori N. Pseudomembranous colitis due to intestinal amebiasis. Intern Med. 2021;60(20):3335–3336. doi:10.2169/internalmedicine.7230-21
14. Ford AC, Sperber AD, Corsetti M, et al. Irritable bowel syndrome. Lancet. 2020;396(10263):1675–1688. doi:10.1016/s0140-6736(20)31548-8
15. Flynn S, Eisenstein S. Inflammatory bowel disease presentation and diagnosis. Surg Clin North Am. 2019;99(6):1051–1062. doi:10.1016/j.suc.2019.08.001
16. Shawki S, Ashburn J, Signs SA, et al. Colon cancer: inflammation-associated cancer. Surg Oncol Clin N Am. 2018;27(2):269–287. doi:10.1016/j.soc.2017.11.003
17. Yang N, Ruan M, Jin S. Melanosis coli: a comprehensive review. Gastroenterol Hepatol. 2020;43(5):266–272. doi:10.1016/j.gastrohep.2020.01.002
18. Porter RJ, Arends MJ, Churchhouse AMD, et al. Inflammatory bowel disease-associated colorectal cancer: translational risks from mechanisms to medicines. J Crohns Colitis. 2021;15(12):2131–2141. doi:10.1093/ecco-jcc/jjab102
19. Caioni G, Viscido A, d’Angelo M, et al. Inflammatory bowel disease: new insights into the interplay between environmental factors and PPARγ. Int J Mol Sci. 2021;22(3):985. doi:10.3390/ijms22030985
20. Woźniak D, Cichy W, Przysławski J, et al. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv Med Sci. 2021;66(2):284–292. doi:10.1016/j.advms.2021.05.003
21. Zhang Y, Yu X, Yu E, et al. Changes in gut microbiota and plasma inflammatory factors across the stages of colorectal tumorigenesis: a case-control study. BMC Microbiol. 2018;18(1):92. doi:10.1186/s12866-018-1232-6
22. Tortora SC, Bodiwala VM, Quinn A, et al. Microbiome and colorectal carcinogenesis: linked mechanisms and racial differences. World J Gastrointest Oncol. 2022;14(2):375–395. doi:10.4251/wjgo.v14.i2.375
23. Dai ZF, Ma XY, Yang RL, et al. Intestinal flora alterations in patients with ulcerative colitis and their association with inflammation. Exp Ther Med. 2021;22(5):1322. doi:10.3892/etm.2021.10757
24. Farooq RK, Alamoudi W, Alhibshi A, et al. Varied composition and underlying mechanisms of gut microbiome in neuroinflammation. Microorganisms. 2022;10(4):705. doi:10.3390/microorganisms10040705
25. Zhou B, Yuan Y, Zhang S, et al. Intestinal flora and disease mutually shape the regional immune system in the intestinal tract. Front Immunol. 2020;11:575. doi:10.3389/fimmu.2020.00575
26. Xu F, Fu Y, Sun TY, et al. The interplay between host genetics and the gut microbiome reveals common and distinct microbiome features for complex human diseases. Microbiome. 2020;8(1):145. doi:10.1186/s40168-020-00923-9
27. Valdes AM, Walter J, Segal E, et al. Role of the gut microbiota in nutrition and health. BMJ. 2018:k2179. doi:10.1136/bmj.k2179
28. Nagpal R, Yadav H, Marotta F. Gut microbiota: the next-gen frontier in preventive and therapeutic medicine? Front Med. 2014;1:15. doi:10.3389/fmed.2014.00015
29. Cheng WY, Wu CY, Yu J. The role of gut microbiota in cancer treatment: friend or foe? Gut. 2020;69(10):1867–1876. doi:10.1136/gutjnl-2020-321153
30. Yue B, Yu ZL, Lv C, et al. Regulation of the intestinal microbiota: an emerging therapeutic strategy for inflammatory bowel disease. World J Gastroenterol. 2020;26(30):4378–4393. doi:10.3748/wjg.v26.i30.4378
31. Reimer RA. Establishing the role of diet in the microbiota-disease axis. Nat Rev Gastroenterol Hepatol. 2019;16(2):86–87. doi:10.1038/s41575-018-0093-7
32. Wu GD, Chen J, Hoffmann C, et al. Linking long-term dietary patterns with gut microbial enterotypes. Science. 2011;334(6052):105–108. doi:10.1126/science.1208344
33. Vindigni SM, Surawicz CM. Fecal microbiota transplantation. Gastroenterol Clin North Am. 2017;46(1):171–185. doi:10.1016/j.gtc.2016.09.012
34. Tkach S, Dorofeyev A, Kuzenko I, et al. Current status and future therapeutic options for fecal microbiota transplantation. Medicina. 2022;58(1):84. doi:10.3390/medicina58010084
35. Marasco G, Cirota GG, Rossini B, et al. Probiotics, prebiotics and other dietary supplements for gut microbiota modulation in celiac disease patients. Nutrients. 2020;12(9):2674. doi:10.3390/nu12092674
36. Tsai Y-L, Lin T-L, Chang C-J, et al. Probiotics, prebiotics and amelioration of diseases. J Biomed Sci. 2019;26(1):3. doi:10.1186/s12929-018-0493-6
37. Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell. 2018;174(6):1388–1405.e1321. doi:10.1016/j.cell.2018.08.041
38. Snelson M, Kellow NJ, Coughlan MT. Modulation of the gut microbiota by resistant starch as a treatment of chronic kidney diseases: evidence of efficacy and mechanistic insights. Adv Nutr. 2019;10(2):303–320. doi:10.1093/advances/nmy068
39. Gibson GR, Beatty ER, Wang X, et al. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108(4):975–982. doi:10.1016/0016-5085(95
40. Gibson GR, Hutkins R, Sanders ME, et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502. doi:10.1038/nrgastro.2017.75
41. Colantonio AG, Werner SL, Brown M. The Effects of prebiotics and substances with prebiotic properties on metabolic and inflammatory biomarkers in individuals with type 2 diabetes mellitus: a systematic review. J Acad Nutr Diet. 2020;120(4):587–607e582. doi:10.1016/j.jand.2018.12.013
42. Saulnier DM, Molenaar D, de Vos WM, et al. Identification of prebiotic fructooligosaccharide metabolism in lactobacillus plantarum WCFS1 through microarrays. Appl Environ Microbiol. 2007;73(6):1753–1765. doi:10.1128/aem.01151-06
43. Gyawali R, Nwamaioha N, Fiagbor R, et al. The role of prebiotics in disease prevention and health promotion. In Watson RR, Preedy VR, editors. Dietary Interventions in Gastrointestinal Diseases. Academic Press; 2019. 151–167.
44. Le Bastard Q, Chapelet G, Javaudin F, et al. The effects of inulin on gut microbial composition: a systematic review of evidence from human studies. Eur J Clin Microbiol Infect Dis. 2020;39(3):403–413. doi:10.1007/s10096-019-03721-w
45. Chen Z, Lin S, Jiang Y, et al. Effects of bread yeast cell wall beta-glucans on mice with loperamide-induced constipation. J Med Food. 2019;22(10):1009–1021. doi:10.1089/jmf.2019.4407
46. Zaman SA, Sarbini SR. The potential of resistant starch as a prebiotic. Crit Rev Biotechnol. 2016;36(3):578–584. doi:10.3109/07388551.2014.993590
47. Sliżewska K. The citric acid-modified, enzyme-resistant dextrin from potato starch as a potential prebiotic. Acta Biochim Pol. 2013;60(4):671–675. doi:10.18388/ABP.2013_2039
48. Chen G, Wang M, Zeng Z, et al. Fuzhuan brick tea polysaccharides serve as a promising candidate for remodeling the gut microbiota from colitis subjects in vitro: fermentation characteristic and anti-inflammatory activity. Food Chem. 2022;391:133203. doi:10.1016/j.foodchem.2022.133203
49. Vázquez MJ, Alonso JL, Domı́nguez H, et al. Xylooligosaccharides: manufacture and applications. Trends Food Sci Technol. 2000;11(11):387–393. doi:10.1016/S0924-2244(01)00031-0
50. Torres DPM, Gonçalves M, Teixeira JA, et al. Galacto-oligosaccharides: production, properties, applications, and significance as prebiotics. Compr Rev Food Sci Food Saf. 2010;9(5):438–454. doi:10.1111/j.1541-4337.2010.00119.x
51. Singh DP, Singh J, Boparai RK, et al. Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol Res. 2017;123:103–113. doi:10.1016/j.phrs.2017.06.015
52. Wan J, Zhang J, Chen D, et al. Alginate oligosaccharide enhances intestinal integrity of weaned pigs through altering intestinal inflammatory responses and antioxidant status. RSC Adv. 2018;8(24):13482–13492. doi:10.1039/c8ra01943f
53. You L, Gong Y, Li L, et al. Beneficial effects of three brown seaweed polysaccharides on gut microbiota and their structural characteristics: an overview. Int J Food Sci Technol. 2019;55:1199–1206. doi:10.1111/ijfs.14408
54. Jun X, Haiyan L, Chenjian L. Study on the physiological functions of soybean oligosaccharides from traditional fermented soybean food. Chinese Journal of Microecology. 2010; 2010:2277–2284.
55. Wang H, Zhang X, Wang S, et al. Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food Funct. 2018;9(7):3916–3929. doi:10.1039/c8fo00209f
56. Muanprasat C, Chatsudthipong V. Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol Ther. 2017;170:80–97. doi:10.1016/j.pharmthera.2016.10.013
57. Ai T, Hao L, Shang L, et al. Konjac oligosaccharides modulate the gut environment and promote bone health in calcium-deficient mice. J Agric Food Chem. 2021;69(15):4412–4422. doi:10.1021/acs.jafc.0c07839
58. Masi AC, Embleton ND, Lamb CA, et al. Human milk oligosaccharide DSLNT and gut microbiome in preterm infants predicts necrotising enterocolitis. Gut. 2021;70(12):2273–2282. doi:10.1136/gutjnl-2020-322771
59. Vandeputte D, Falony G, Vieira-Silva S, et al. Prebiotic inulin-type fructans induce specific changes in the human gut microbiota. Gut. 2017;66(11):1968–1974. doi:10.1136/gutjnl-2016-313271
60. Song C, Huang F, Liu L, et al. Characterization and prebiotic properties of pectin polysaccharide from Clausena lansium (Lour.) Skeels fruit. Int J Biol Macromol. 2022;194:412–421. doi:10.1016/j.ijbiomac.2021.11.083
61. Trivieri N, Panebianco C, Villani A, et al. High levels of prebiotic resistant starch in diet modulate a specific pattern of miRNAs expression profile associated to a better overall survival in pancreatic cancer. Biomolecules. 2020;11(1):26. doi:10.3390/biom11010026
62. Rani A, Baruah R, Goyal A. Prebiotic chondroitin sulfate disaccharide isolated from chicken keel bone exhibiting anticancer potential against human colon cancer cells. Nutr Cancer. 2019;71(5):825–839. doi:10.1080/01635581.2018.1521446
63. Gholizadeh Shamasbi S, Dehgan P, Mohammad-Alizadeh Charandabi S, et al. The effect of resistant dextrin as a prebiotic on metabolic parameters and androgen level in women with polycystic ovarian syndrome: a randomized, triple-blind, controlled, clinical trial. Eur J Nutr. 2019;58(2):629–640. doi:10.1007/s00394-018-1648-7
64. Liu L, He Y, Wang K, et al. Metagenomics approach to the intestinal microbiome structure and function in high fat diet-induced obesity in mice fed with conjugated linoleic acid (CLA). Food Funct. 2020;11(11):9729–9739. doi:10.1039/d0fo02112a
65. Costantini L, Molinari R, Farinon B, et al. Impact of omega-3 fatty acids on the gut microbiota. Int J Mol Sci. 2017;18(12):2645. doi:10.3390/ijms18122645
66. Shi T, Bian X, Yao Z, et al. Quercetin improves gut dysbiosis in antibiotic-treated mice. Food Funct. 2020;11(9):8003–8013. doi:10.1039/d0fo01439g
67. Montella R, Coïsson JD, Travaglia F, et al. Bioactive compounds from hazelnut skin (Corylus avellana L.): effects on Lactobacillus plantarum P17630 and Lactobacillus crispatus P17631. J Funct Foods. 2013;5(1):306–315. doi:10.1016/j.jff.2012.11.001
68. Phuriyakorn S, Seechamnanturakit V, Wichienchot S. Antioxidant and prebiotic gut-microbiota effects of dietary phenolic compounds in Etlingera elatior extracts. Int Food Res J. 2019;26(6):1751–1761.
69. Calderón-Pérez L, Llauradó E, Companys J, et al. Interplay between dietary phenolic compound intake and the human gut microbiome in hypertension: a cross-sectional study. Food Chem. 2021;344:128567. doi:10.1016/j.foodchem.2020.128567
70. Ortega-Gonzalez M, Ocon B, Romero-Calvo I, et al. Nondigestible oligosaccharides exert nonprebiotic effects on intestinal epithelial cells enhancing the immune response via activation of TLR4-NFkappaB. Mol Nutr Food Res. 2014;58(2):384–393. doi:10.1002/mnfr.201300296
71. Réquilé M, Gonzàlez Alvarez DO, Delanaud S, et al. Use of a combination of in vitro models to investigate the impact of chlorpyrifos and inulin on the intestinal microbiota and the permeability of the intestinal mucosa. Environ Sci Pollut Res Int. 2018;25(23):22529–22540. doi:10.1007/s11356-018-2332-4
72. de Kivit S, Saeland E, Kraneveld AD, et al. Galectin-9 induced by dietary synbiotics is involved in suppression of allergic symptoms in mice and humans. Allergy. 2012;67(3):343–352. doi:10.1111/j.1398-9995.2011.02771.x
73. Brosseau C, Selle A, Palmer DJ, et al. Prebiotics: mechanisms and preventive effects in allergy. Nutrients. 2019;11(8):1841. doi:10.3390/nu11081841
74. den Besten G, van Eunen K, Groen AK, et al. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res. 2013;54(9):2325–2340. doi:10.1194/jlr.R036012
75. Martin-Gallausiaux C, Marinelli L, Blottière HM, et al. SCFA: mechanisms and functional importance in the gut. Proc Nutr Soc. 2021;80(1):37–49. doi:10.1017/s0029665120006916
76. Corrêa-Oliveira R, Fachi JL, Vieira A, et al. Regulation of immune cell function by short-chain fatty acids. Clin Transl Immunology. 2016;5(4):e73. doi:10.1038/cti.2016.17
77. van der Hee B, Wells JM. Microbial regulation of host physiology by short-chain fatty acids. Trends Microbiol. 2021;29(8):700–712. doi:10.1016/j.tim.2021.02.001
78. Serino M. SCFAs – The thin microbial metabolic line between good and bad. Nat Rev Endocrinol. 2019;15(6):318–319. doi:10.1038/s41574-019-0205-7
79. Byrne CS, Chambers ES, Morrison DJ, et al. The role of short chain fatty acids in appetite regulation and energy homeostasis. Int J Obes (Lond). 2015;39(9):1331–1338. doi:10.1038/ijo.2015.84
80. Yao Y, Cai X, Fei W, et al. The role of short-chain fatty acids in immunity, inflammation and metabolism. Crit Rev Food Sci Nutr. 2022;62(1):1–12. doi:10.1080/10408398.2020.1854675
81. Keppler K, Humpf HU. Metabolism of anthocyanins and their phenolic degradation products by the intestinal microflora. Bioorg Med Chem. 2005;13(17):5195–5205. doi:10.1016/j.bmc.2005.05.003
82. Ohno M, Nishida A, Sugitani Y, et al. Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS One. 2017;12(10):e0185999. doi:10.1371/journal.pone.0185999
83. Zheng H, Powell JE, Steele MI, et al. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc Natl Acad Sci U S A. 2017;114(18):4775–4780. doi:10.1073/pnas.1701819114
84. Chiu HI, Lim V. Wheat germ agglutinin-conjugated disulfide cross-linked alginate nanoparticles as a docetaxel carrier for colon cancer therapy. Int J Nanomedicine. 2021;16:2995–3020. doi:10.2147/ijn.S302238
85. Ju S, Ge Y, Li P, et al. Dietary quercetin ameliorates experimental colitis in mouse by remodeling the function of colonic macrophages via a heme oxygenase-1-dependent pathway. Cell Cycle. 2018;17(1):53–63. doi:10.1080/15384101.2017.1387701
86. Caddeo C, Nácher A, Díez-Sales O, et al. Chitosan-xanthan gum microparticle-based oral tablet for colon-targeted and sustained delivery of quercetin. J Microencapsul. 2014;31(7):694–699. doi:10.3109/02652048.2014.913726
87. Kennel KB, Greten FR. Immune cell – produced ROS and their impact on tumor growth and metastasis. Redox Biol. 2021;42:101891. doi:10.1016/j.redox.2021.101891
88. Sun Q, Luan L, Arif M, et al. Redox-sensitive nanoparticles based on 4-aminothiophenol-carboxymethyl inulin conjugate for budesonide delivery in inflammatory bowel diseases. Carbohydr Polym. 2018;189:352–359. doi:10.1016/j.carbpol.2017.12.021
89. Nunthanid J, Huanbutta K, Luangtana-Anan M, et al. Development of time-, pH-, and enzyme-controlled colonic drug delivery using spray-dried chitosan acetate and hydroxypropyl methylcellulose. Eur J Pharm Biopharm. 2008;68(2):253–259. doi:10.1016/j.ejpb.2007.05.017
90. Zhang L, Sang Y, Feng J, et al. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J Drug Target. 2016;24(7):579–589. doi:10.3109/1061186X.2015.1128941
91. Cui M, Zhang M, Liu K. Colon-targeted drug delivery of polysaccharide-based nanocarriers for synergistic treatment of inflammatory bowel disease: a review. Carbohydr Polym. 2021;272:118530. doi:10.1016/j.carbpol.2021.118530
92. Zheng DW, Li RQ, An JX, et al. Prebiotics-encapsulated probiotic spores regulate gut microbiota and suppress colon cancer. Adv Mater. 2020;32(45):e2004529. doi:10.1002/adma.202004529
93. Wen P, Hu TG, Li L, et al. A colon-specific delivery system for quercetin with enhanced cancer prevention based on co-axial electrospinning. Food Funct. 2018;9(11):5999–6009. doi:10.1039/c8fo01216d
94. Castangia I, Nácher A, Caddeo C, et al. Therapeutic efficacy of quercetin enzyme-responsive nanovesicles for the treatment of experimental colitis in rats. Acta Biomater. 2015;13:216–227. doi:10.1016/j.actbio.2014.11.017
95. Xu C, Chen S, Chen C, et al. Colon-targeted oral nanoparticles based on ROS-scavenging hydroxyethyl starch-curcumin conjugates for efficient inflammatory bowel disease therapy. Int J Pharm. 2022;623:121884. doi:10.1016/j.ijpharm.2022.121884
96. Fares MM, Salem MS. Dissolution enhancement of curcumin via curcumin-prebiotic inulin nanoparticles. Drug Dev Ind Pharm. 2015;41(11):1785–1792. doi:10.3109/03639045.2015.1004184
97. Shivhare K, Garg C, Priyam A, et al. Enzyme sensitive smart inulin-dehydropeptide conjugate self-assembles into nanostructures useful for targeted delivery of ornidazole. Int J Biol Macromol. 2018;106:775–783. doi:10.1016/j.ijbiomac.2017.08.071
98. Bondarenko OM, Ivask A, Kahru A, et al. Bacterial polysaccharide levan as stabilizing, non-toxic and functional coating material for microelement-nanoparticles. Carbohydr Polym. 2016;136:710–720. doi:10.1016/j.carbpol.2015.09.093
99. Jing W, Zhu M, Wang F, et al. Hyaluronic acid-melatonin nanoparticles improve the dysregulated intestinal barrier, microbiome and immune response in mice with dextran sodium sulfate-induced colitis. J Biomed Nanotechnol. 2022;18(1):175–184. doi:10.1166/jbn.2022.3232
100. Chuah LH, Roberts CJ, Billa N, et al. Cellular uptake and anticancer effects of mucoadhesive curcumin-containing chitosan nanoparticles. Colloids Surf B Biointerfaces. 2014;116:228–236. doi:10.1016/j.colsurfb.2014.01.007
101. Dube A, Nicolazzo JA, Larson I. Chitosan nanoparticles enhance the plasma exposure of (-)-epigallocatechin gallate in mice through an enhancement in intestinal stability. Eur J Pharm Sci. 2011;44(3):422–426. doi:10.1016/j.ejps.2011.09.004
102. Alkhader E, Roberts CJ, Rosli R, et al. Pharmacokinetic and anti-colon cancer properties of curcumin-containing chitosan-pectinate composite nanoparticles. J Biomater Sci Polym Ed. 2018;29(18):2281–2298. doi:10.1080/09205063.2018.1541500
103. Boni FI, Almeida A, Lechanteur A, et al. Mucoadhesive nanostructured polyelectrolytes complexes modulate the intestinal permeability of methotrexate. Eur J Pharm Sci. 2018;111:73–82. doi:10.1016/j.ejps.2017.09.042
104. Samprasit W, Opanasopit P, Chamsai B. Mucoadhesive chitosan and thiolated chitosan nanoparticles containing alpha mangostin for possible Colon-targeted delivery. Pharm Dev Technol. 2021;26(3):362–372. doi:10.1080/10837450.2021.1873370
105. Singh S, Kotla NG, Tomar S, et al. A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. Int J Nanomedicine. 2015;10:7175–7182. doi:10.2147/IJN.S89030
106. Zheng DW, Dong X, Pan P, et al. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat Biomed Eng. 2019;3(9):717–728. doi:10.1038/s41551-019-0423-2
107. Wen Y, Wen P, Hu TG, et al. Encapsulation of phycocyanin by prebiotics and polysaccharides-based electrospun fibers and improved colon cancer prevention effects. Int J Biol Macromol. 2020;149:672–681. doi:10.1016/j.ijbiomac.2020.01.189
108. Chandrarathna H, Liyanage TD, Edirisinghe SL, et al. Marine microalgae, spirulina maxima-derived modified pectin and modified pectin nanoparticles modulate the gut microbiota and trigger immune responses in mice. Mar Drugs. 2020;18(3):175. doi:10.3390/md18030175
109. Bai X, Feng Z, Peng S, et al. Chitosan-modified Phellinus igniarius polysaccharide PLGA nanoparticles ameliorated inflammatory bowel disease. Biomater Adv. 2022;139:213002. doi:10.1016/j.bioadv.2022.213002
110. Zhang X, Ma Y, Ma L, et al. Oral administration of chondroitin sulfate-functionalized nanoparticles for colonic macrophage-targeted drug delivery. Carbohydr Polym. 2019;223:115126. doi:10.1016/j.carbpol.2019.115126
111. Song Q, Jia J, Niu X, et al. An oral drug delivery system with programmed drug release and imaging properties for orthotopic colon cancer therapy. Nanoscale. 2019;11(34):15958–15970. doi:10.1039/c9nr03802g
112. Hou Y, Jin J, Duan H, et al. Targeted therapeutic effects of oral inulin-modified double-layered nanoparticles containing chemotherapeutics on orthotopic colon cancer. Biomaterials. 2022;283:121440. doi:10.1016/j.biomaterials.2022.121440
113. Yang L. Biorelevant dissolution testing of colon-specific delivery systems activated by colonic microflora. J Control Release. 2008;125(2):77–86. doi:10.1016/j.jconrel.2007.10.026
114. Bayat A, Dorkoosh FA, Dehpour AR, et al. Nanoparticles of quaternized chitosan derivatives as a carrier for colon delivery of insulin: ex vivo and in vivo studies. Int J Pharm. 2008;356(1–2):259–266. doi:10.1016/j.ijpharm.2007.12.037
115. Badve SS, Sher P, Korde A, et al. Development of hollow/porous calcium pectinate beads for floating-pulsatile drug delivery. Eur J Pharm Biopharm. 2007;65(1):85–93. doi:10.1016/j.ejpb.2006.07.010
116. Sun P, Chuan-Jiang MA, Liu LY. Pharmacokinetic and intestinal absorption study on rats of emodin in colon-targeted huchang qingdu pellets. Chin Med Infor Tradit Chin Med. 2015;22(9):79–84.
117. McClements DJ, Xiao H. Is nano safe in foods? Establishing the factors impacting the gastrointestinal fate and toxicity of organic and inorganic food-grade nanoparticles. NPJ Sci Food. 2017;1:6. doi:10.1038/s41538-017-0005-1
118. Araújo F, Shrestha N, Granja PL, et al. Safety and toxicity concerns of orally delivered nanoparticles as drug carriers. Expert Opin Drug Metab Toxicol. 2015;11(3):381–393. doi:10.1517/17425255.2015.992781



