Nanomedicines for various diseases are in development – but research facilities produce vastly inconsistent results on how the body will react to them
Nanomedicines took the spotlight during the COVID-19 pandemic. Researchers are using these very small and intricate materials to develop diagnostic tests and treatments. Nanomedicine is already used for various diseases, such as the COVID-19 vaccines and therapies for cardiovascular disease. The “nano” refers to the use of particles that are only a few hundred nanometers in size, which is significantly smaller than the width of a human hair.
Although researchers have developed several methods to improve the reliability of nanotechnologies, the field still faces one major roadblock: a lack of a standardized way to analyze biological identity, or how the body will react to nanomedicines. This is essential information in evaluating how effective and safe new treatments are.
I’m a researcher studying overlooked factors in nanomedicine development. In our recently published research, my colleagues and I found that analyses of biological identity are highly inconsistent across proteomics facilities that specialize in studying proteins.
Inconsistent results
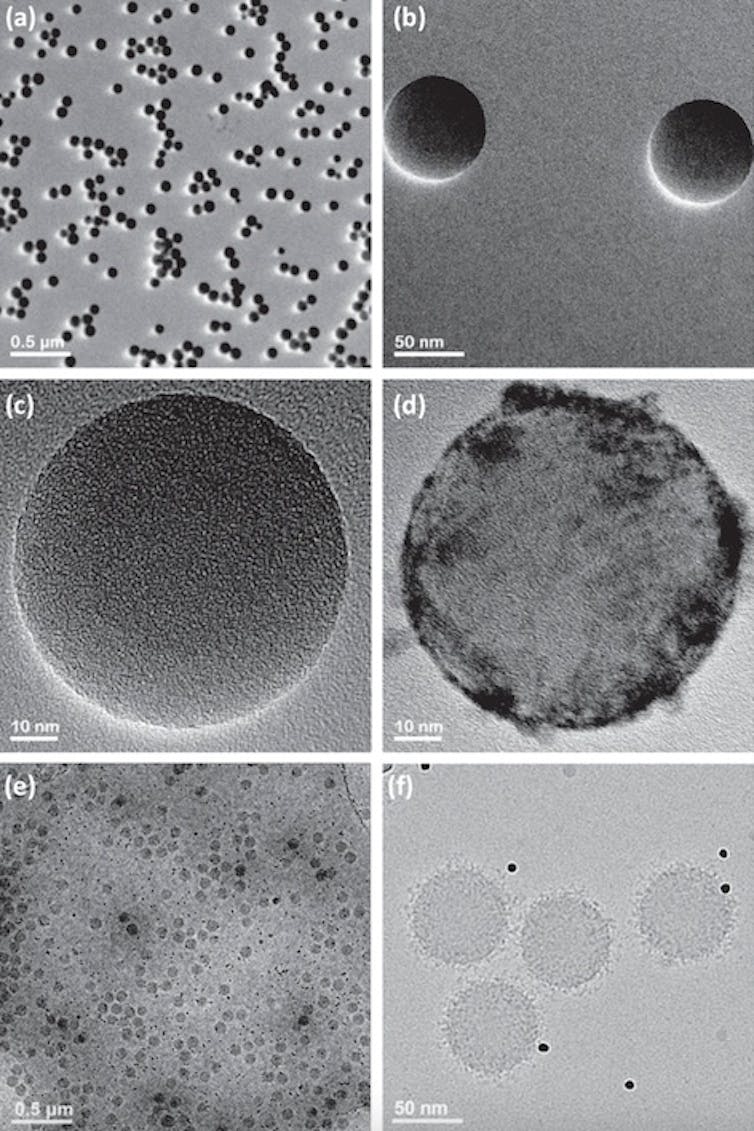
Nanomedicines, just like with all medications, are surrounded by proteins from the body once they come into contact with the bloodstream. This protein coating, known as a protein corona, gives nanoparticles a biological identity that determines how the body will recognize and interact with it, like how the immune system has specific reactions against certain pathogens and allergens.
Knowing the precise type, amount and configuration of the proteins and other biomolecules attached to the surface of nanomedicines is critical to determine safe and effective dosages for treatments. However, one of the few available approaches to analyze the composition of protein coronas requires instruments that many nanomedicine laboratories lack. So these labs typically send their samples to separate proteomics facilities to do the analysis for them. Unfortunately, many facilities use different sample preparation methods and instruments, which can lead to differences in results.

Ashkarran et al. (2022)/Nature Communications, CC BY
We wanted to test how consistently these proteomics facilities analyzed protein corona samples. To do this, my colleagues and I sent biologically identical protein coronas to 17 different labs in the U.S. for analysis.
We had striking results: Less than 2% of the proteins the labs identified were the same.
Our results reveal an extreme lack of consistency in the analyses researchers use to understand how nanomedicines work in the body. This may pose a significant challenge not only to ensuring the accuracy of diagnostics, but also the effectiveness and safety of treatments based on nanomedicines.
Why standardize nanomedicine?
Researchers have been working to improve the safety and efficacy of nanomedicine through various approaches. These include modifying study protocols, methodologies and analytical techniques to standardize the field and improve the reliability of nanomedicine data.
Aligned with these efforts, my team and I have identified several critical but often overlooked factors that can influence the performance of a nanomedicine, such as a person’s sex, prior medical conditions and disease type. Taking these factors into account when designing studies and interpreting results could enable researchers to produce more reliable and accurate data and lead to better nanomedicine treatments.

