In the core of the cell: New insights into the utilization of nanotechnology-based drugs

Novel drugs, such as vaccines against COVID-19, among others, are based on drug transport using nanoparticles. Whether this drug transport is negatively influenced by an accumulation of blood proteins on the nanoparticle’s surface was not clarified for a long time.
Scientists at the Max Planck Institute for Polymer Research have now followed the path of such a particle into a cell using a combination of several microscopy methods. They were able to observe a cell-internal process that effectively separates blood components and nanoparticles.
Nanoparticles are a current field of research and it is impossible to imagine modern medicine without them. They serve as microscopic drug capsules that are less than a thousandth of a millimeter in diameter. Among other things, they are used in current vaccines against COVID-19 to effectively deliver active ingredients to where they are actually needed. In most cases, the capsules dock onto cells, are enveloped by them, and are absorbed into them. Inside the cell, chemical processes can then open the capsules, releasing the active ingredient.
However, this idealized process usually does not take place: As it travels through the bloodstream, blood proteins accumulate on the surface of the nanotransporter. These also find their way into the cell. For a long time, it was an unresolved question whether this process impairs the release of the active ingredient.
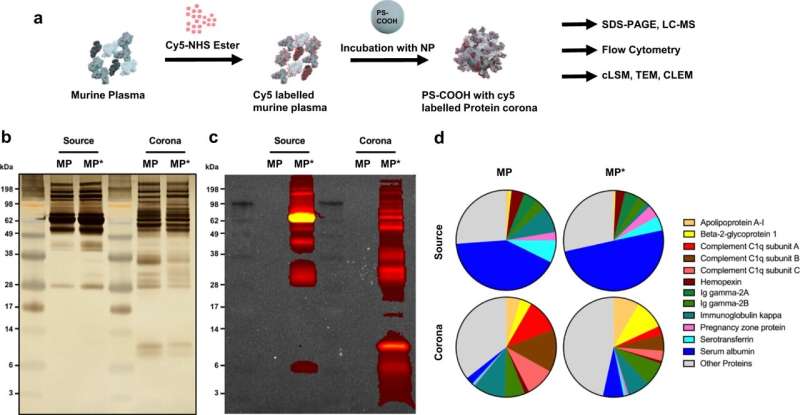
Scientists working with Ingo Lieberwirth, group leader in Katharina Landfester’s department, have now addressed this question. They have labeled a nanoparticle and blood proteins with different fluorescent dyes. As a result, both glow with different colors when viewed through a high-resolution light microscope. At the same time, the researchers were able to observe the process in parallel and at higher resolution using an electron microscope.
By combining both methods, the scientists were able to observe that the cell initially absorbs the composite of nanoparticles and blood proteins. In the cell, they now observed something surprising: The protein coating detaches from the nanoparticle and releases it. After some time, proteins and particles are present separately in the cell.
“We therefore assume that the drug release in the cell is not disturbed by the protein coating,” says Ingo Lieberwirth. “However, it is now important to find out how exactly the process takes place inside the cell.”
The scientists have now published their results in Nature Communications.
More information:
Shen Han et al, Endosomal sorting results in a selective separation of the protein corona from nanoparticles, Nature Communications (2023). DOI: 10.1038/s41467-023-35902-9
Provided by
Max Planck Society
Citation:
In the core of the cell: New insights into the utilization of nanotechnology-based drugs (2023, January 20)
retrieved 20 January 2023
from https://phys.org/news/2023-01-core-cell-insights-nanotechnology-based-drugs.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.



