Anti-Oxidative, Anti-Apoptotic, and M2 Polarized DSPC Liposome Nanoparticles for Selective Treatment of Atherosclerosis
Introduction
Atherosclerosis is the main cause of coronary heart disease, stroke, and peripheral vascular disease. At present, the efficacy of drugs used in prevention and treatment is very limited, and there are many adverse reactions. Atherosclerosis is accelerated by many factors, such as the production of reactive oxygen species (ROS).1 Oxidative stress is one of the main pathogenic elements of atherosclerosis. Statins are the most commonly used drugs to regulate blood lipids in clinical applications. Their side effects are the most common such as serious muscle toxicity and liver toxicity. However, small molecule antioxidants have not brought positive effects on the prevention and treatment of atherosclerosis. Therefore, exploring new antioxidant strategies has become the key to preventing oxidative stress.
Epigallocatechin gallate (EGCG) is the main component of green tea polyphenols. It is a catechin monomer isolated from tea. It has antibacterial, antiviral, antioxidant, anti-arteriosclerosis, antithrombotic, anti-vascular proliferation, anti-inflammatory, and anti-tumor effects. Bernhard Hennig proved that pretreatment with EGCG blocked fatty acid-induced caveolin-1 and COX-2 expression in a concentration-dependent manner.2 Their data provided evidence that EGCG may play a critical role in regulating vascular endothelial cell activation and protection. Another study shows EGCG controlled many cardiovascular health-promoting activities by regulating various pathways. EGCG has a wide range of therapeutic effects, including anti-atherosclerosis, anti-inflammatory, and antioxidant functions.3 These therapeutic effects are mainly related to the inhibition of low-density lipoprotein cholesterol, the reduction of inflammatory markers, and the inhibition of ROS and cell apoptosis.4 Animal studies proved that EGCG can reduce the markers of atherosclerosis and lipid peroxidation, especially the concentration of oxidized low-density lipoprotein and malondialdehyde, and also reduce postprandial blood lipids in hypercholesterolemic subjects.5
Macrophage function in atherosclerosis is also important. Macrophages can mainly differentiate into M1 macrophages and M2 macrophages through different activation pathways.6,7 The two cell subtypes have great differences in function. M1 macrophages are mostly involved in inflammation-related diseases, such as colitis, viral myocarditis, arthritis, and acute peritonitis, and release a large number of pro-inflammatory cytokines, causing serious inflammatory damage in the lesions. M2 macrophages are mostly involved in tissue healing and angiogenesis. M2 macrophages create an immunosuppressive environment by releasing anti-inflammatory cytokines and inhibiting the immune effect of T cells. Feiyang Cai’s group showed that EGCG inhibited the polarization of M1 macrophages, but promoted the polarization of M2 macrophages. These effects may be because EGCG inhibits macrophage nuclear factor- κB signal and glycolysis.8 Several studies support that EGCG has several benefits for heart diseases and obesity.9 Nevertheless, EGCG is not very stable in water and physiological solutions which has low bioavailability and no target specificity and is easily metabolized by enzymes in the liver, kidneys, and other tissues.10 It constitutes the main drawback to using this molecule in prevention and therapy. Nanotechnology is an ideal solution that may increase the applicability of EGCG to atherosclerotic treatment.11
Liposomes are particles composed of the lipid bilayer, which can mediate the passage of drugs through the cell membrane. Liposomes have many advantages, including lymphatic targeting, and are used as passive targeting materials for the liver spleen reticular system.12 For the treatment of parasitic diseases and mononuclear macrophage systems, liposome formulation is a valuable approach.13 Liposomes have a sustained-release effect, which can slow-release, and delay renal excretion and metabolism, thus prolonging the time of drug action.14 Liposomes can also reduce the toxicity of drugs, such as amphotericin B liposomes can effectively reduce the toxicity of the heart. Liposomes improve the stability of the drug, such as insulin liposomes, vaccines, etc., which can significantly improve the drug effect.15 In addition, liposomes act as cholesterol receptors and mobilize cholesterol deposition from atherosclerotic arteries to the liver for excretion.16 The scientific and clinical evidence are relating to the use of liposomes in the diagnosis and management of AS.17 Kee Woei Ng’s group indicated that cationic liposomes and distearyl phosphatidylcholine (DSPC) liposomes were internalized more by both macrophages and foam cells.
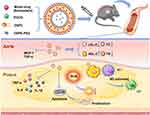
In this study, we addressed the oxidative stress and inflammation issue of atherosclerosis by developing a stable, intravenously injectable SIM and EGCG-loaded liposome nanoparticles (SE-LNPs) that exhibit a sustained release profile, potentially enabling the accumulation of the majority amount of drug at the targeted atherosclerotic plaque. We investigated the anti-oxidative and anti-apoptotic effects of the formulation in vitro. Furthermore, we assessed the accumulation of SE-LNPs in the atherosclerotic plaque in vivo. Eventually, the therapeutic effect of SE-LNPs was assessed in the ApoE−/− mice model of atherosclerosis. Studies have shown that it is a reliable and selective treatment for atherosclerosis.
Materials and Methods
Preparation of SE-LNPs
The liposomes were composed of DSPC (Sigma, P1138, Thermo Fisher Scientific, USA), and 1,2- distearoyl- sn-glycero −3-phosphoethanolamine- N- [methoxy (polyethylene glycol)-2000] (ammonium salt) (DSPE–PEG2000) (Sigma, 880127, Thermo Fisher Scientific, USA) and other phospholipid excipients. The liposomes were prepared by a thin-film hydration approach.18 Briefly, phospholipids and model drug Simvastatin (SIM, 10 mg) and antioxidant EGCG (1 mg) were weighed and dissolved in chloroform/methanol (2:1, vol/vol) regent in a round bottom flask. The volatilizable regent was removed by rotary evaporator (N-1300D-WB, EYELA, Japan) at 50 °C. Then, the flask was rotated at 100 rpm for 1 h. The drug-loaded lipid film yielded was subsequently hydrated with phosphate-buffered saline (PBS) (0.01M, pH 7.4) at 60 °C to form multilamellar vesicles (MLVs). The MLVs were extruded (10 times) through polycarbonate filters (0.4 µm) fitted on a benchtop extruder (Avestin, LF-1, Ottawa, Canada) to form the liposome nanoparticles (SE-LNPs). Rhodamine-DPPE (Rho) (Sigma, Atto 594 DPPE, 40924, Thermo Fisher Scientific, USA) (0.1 mol%) was used for imaging by the same preparation.
Characterization of SE-LNPs
200 μL fresh prepared SE-LNPs were diluted to 1 mL, added to the standard sample pool, and tested on the machine; The measurement conditions were: the balance time was 1 min, the measurement interval was 10s, and the measurement temperature was 25°C. Replaced the special sample cell for Zeta potential measurement.
5 mg SE-LNPs were prepared by cash withdrawal to 0.1 mL, gently dropped onto the copper mesh, dried at room temperature to remove water vapor, and then observed the morphology of nanoparticles with the transmission electron microscope (TEM, HT7700, Hitachi, Japan).
The release behavior was observed in PBS or hydrogen peroxide (H2O2). The SE-LNPs were resuspended in two groups of PBS solution. Three days later, a group of PBS liquid was changed into an H2O2 solution, and the observation was continued. Finally, the drug content in the supernatant was tested.
The drug SIM loading of SE-LNPs was tested by high-performance liquid chromatography (HPLC, Waters2695, USA). Octadecyl-bonded silane was used as filler, and acetonitrile-0.025mol/L sodium dihydrogen phosphate solution (PH 4.5) (65:35) was used as the mobile phase. The detection wavelength was 238 nm.
We also determined the content of EGCG of SE-LNPs. We used reversed phase high-performance liquid chromatography (RP-HPLC, Waters510, USA) for testing. Chromatographic column: L-Bondpak C18; mobile phase: methanol-water glacial acetic acid 18:60:12 (volume ratio), PH 6.2–6.5; mobile phase was filtered before use, degas for 15–20 min; detection wavelength: 280 nm; nanoparticle sample was extracted three times with ethyl acetate, and combined extraction solution for precise determination.
Cell Lines
The macrophages (RAW264.7) and human umbilical vein endothelial cells (HUVEC) were purchased from Procell Life Science&Technology Co.,Ltd.
Cytotoxicity Study of SE-LNPs
The RAW264.7 were treated with different liposome formulations (LNPs, SE-LNPs) and free drugs at different concentrations (12.5, 25, 50, 100 μg/mL) for 24 h. Untreated cells served as a control. The cytotoxicity of liposomes was examined in terms of cell proliferation using MTT methods according to the manufacturer’s instructions. Collected cells in logarithmic phase, adjusted the concentration of cell suspension and added 100 μL to each well / 5000 cells (96-hole flat plate). Incubated with 5% CO2 at 37 °C for 24 h. Added the drug with a concentration gradient of 100 μL per hole and incubated for 24h. Added 20 μL MTT solution (5 mg/mL) into each well and continued to culture for 4 h. Stopped the culture and carefully sucked out the culture medium in the hole. Added 150 μL dimethyl sulfoxide into each hole, placed it on the shaker, and shake it at low speed for 10 min to fully dissolve the crystals. Measured the absorbance value of each hole at OD490 nm.
Phagocytosis Selectivity of SE-LNPs
The RAW264.7 and HUVEC were treated with liposome formulations (Rho-LNPs) and free drugs (Rho) at 100 μg/mL for 2 h. The steps are as follows: The cells were subcultured, observed as logarithmic growth, diluted to105/mL, and inoculated into a confocal dish. After 24 h of culture, the Rho and Rho-LNPs were dissolved in a serum-free medium and cultured for 3 h. The original culture medium was removed. Washed twice. 500 μL of 4% paraformaldehyde was added and fixed at room temperature for 20 min. The supernatant was discarded. Washed 3 times for 5 min each time. At last, added 300 μL DAPI at room temperature for 3 min, and washed with PBS 4 times, 5 min each time. Finally, the laser confocal microscope (LSM900, ZEISS, Germany) was used for observation.
Antioxidation of SE-LNPs
Preparation of 1 mM H2O2 solution: dilute 30% hydrogen peroxide 100 times to prepare 100 mM solution, and then dilute the stock solution 100 times to prepare 1 mM H2O2 solution. The solution is ready for use. Take 0.2 mL of the prepared nano solution with different concentrations (12.5, 25, 50 μg/mL) and put it in 1 mL of PBS solution and H2O2 solution respectively. After 2 hours, investigated whether the nanoparticles can respond to the H2O2 solution. Dissolved the H2O2 detection reagent (Beyotime, S0038) on ice. Added 50 μL sample or standard into the test tube. Added 100 μL H2O2 detection reagent into each test. Shook gently and left at room temperature for 30 min. Immediately measured A560. Calculated the concentration of H2O2 and converted the removal percentage.
Effect of SE-LNPs on Scavenging ROS
2×105/well RAW264.7 cells were inoculated in 12 well plates and cultured overnight to adhere to the wall. Replaced the original culture medium with EGCG and SE-LNPs (100 μg/mL) for 2 h. PMA was used to stimulate cells to produce oxidative stress responses in vitro. Therefore, the research group selected PMA to stimulate cells to produce high-level ROS as the model control group. Discarded the medium, added the medium containing 100 ng/mL PMA and continued to culture for 4 h. Then replace the medium with a 10 μM DCFH-DA serum-free medium that was incubated for 30 min. The cells were washed with PBS 3 times. After the cells were scraped off, they were centrifuged and resuspended with PBS for detection by flow cytometry (BD FACS CantoII, Becton, Dickinson and Company, USA).
Effect of SE-LNPs on Inhibition of Apoptosis
Macrophages engulf a large amount of oxidized low-density lipoprotein to form foam cells and finally form a necrotic core. Both inflammatory microenvironment and high levels of ROS can promote apoptosis. SE-LNPs have ROS scavenging function, so they are expected to reduce ROS-induced apoptosis.
2×105/well RAW264.7 cells were inoculated in 12 well plates and cultured overnight. Replaced the culture medium with 200 μM H2O2, and continued to incubate for 24 h. Then added and incubated with free drug and SE-LNPs (100 μg/mL) for 24 h. Subsequently, a TUNEL staining apoptosis detection kit was added for staining, and flow cytometry was used for observation.
Induction of M2 by SE-LNPs
2×105/well RAW264.7 cells were inoculated in 12 well plates and cultured overnight. Replaced the culture medium with SIM, EGCG, LNPs, and SE-LNPs (100 μg/mL) and continued to incubate for 48 h. IL-4 (20 ng/mL) serves as a positive control. Then CD206 was added for staining. To prevent the binding of a non-specific protein antigen, blocked with the serum to weaken the background coloration. The time of serum blocking could be adjusted, generally 10–30 min. Washed 3 times. The first antibody was incubated for 4°C overnight. Reheated at roommate for 45 min. Washed 4 times. The second antibody was incubated for 1 h. Washed 4 times. Finally, DAPI anti-fluorescence extraction glycerol covered the slides. A laser confocal microscope and flow cytometry was used for observation.
Effect of SE-LNPs on Inhibition of Inflammatory Cytokines
Atherosclerosis is not only accompanied by an increase in ROS level but also triggers an inflammatory response. 2×105/well RAW264.7 cells were inoculated in 12 well plates and cultured overnight. Replaced the culture medium with 200 μM H2O2, 100 ng/mL LPS and continue to incubate for 24 h. Then added and incubated with free drug and SE-LNPs (100 μg/mL) for 24 h. Next, inflammatory cytokines (TNF-α, IL-6, IL-1β, MCP-1) were tested by ELISA. ELISA Steps: coating process (pay attention to setting blank control and negative control): diluted the used antigen 500 times with coating diluent, and added 100 μL antigens to each well 4 °C overnight; Plates were washed 3 times;10% FBS was used to block at 37 °C for 1h; Added the diluted sample into the enzyme-labeled reaction hole, 100 μL per hole; Placed at 37 °C for 1h; Plates were washed 5 times; Enzyme labeled antibody: diluted 500 times 100 μL per hole. Placed at 37°C for 1 h; Plates were washed 6 times; Added substrate solution (currently used and prepared): TMB solution, 100 μL per well. Kept it away from light at 37°C for 3–5 minutes; Added stop liquid 50 μL Stop solution and determined the experimental results within 20 min. Measured at 450 nm wavelength absorption by a microplate reader.
Animal Model Construction and Evaluation
SPF male Apolipoprotein E (ApoE) −/− mice (6–8 weeks old, weight 18–20 g) are derived from the Animal Experiment Center of Southern Hospital, Southern Medical University, approval number: NFYY-2021-0623. Animals comply with the “Regulations on Animal Protection and Use of Southern Hospital of Southern Medical University”.
ApoE −/− mice combined with a high-fat diet (HFD, Cocoa butter, D12108C) were used to induce as the model. They were randomly divided into 4 groups: high-fat diet group (ApoE−/− + HFD), SIM, LNPs, and SE-LNPs. Each group was administered by intravenous injection. After 8 weeks of feeding, the mouse as the model was identified by detecting the general condition, blood lipid level, Oil red O staining of the aorta, and hematoxylin-eosin (HE) staining of aortic pathological sections.
In vivo Therapeutic Study
In the therapeutic study, mice (n=6 per group, 8 weeks on a high-fat diet) were injected intravenously with 0.9% NaCl (control), free SIM (10 mg/kg), EGCG (1 mg/kg), LNPs, SE-LNPs (drug-containing was equal to free SIM) twice a week for three consecutive weeks while maintaining the high-fat diet. Mice were weighed once per week throughout the experiment. The mice were sacrificed 24 h after the last injection. Take 400 μL of whole blood was allowed to stand at room temperature for 30 min, 3500 g was centrifuged for 10 min, and the serum was taken for detection of total cholesterol (TC), triglyceride (TG), high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C). Plasma inflammatory cytokines (TNF-α, IL-6, IL-1β, MCP-1) were tested by ELISA.
Oil Red O Staining of the Aorta
A sampling of the whole aorta: carefully cut the whole aorta together with the heart, and separate the adipose tissue from the aortic root to the perivascular area of the iliac artery under the posture microscope. Cut the blood vessels longitudinally to form a Y-shaped shape and stain with Oil red O for 30 min. After 60% isopropanol washed off the floating color, the high-definition camera took photos.
HE and Masson Staining of the Aortic Root
HE staining of the aortic root: Paraffin sections were dewaxed to water. Put the slices into xylene, gradient concentration (100–70%) ethanol, and distilled water. Hematoxylin staining nucleus: slice into Harris hematoxylin stained for 3–8min, washed with tap water, differentiated with 1% hydrochloric alcohol for several seconds, rinsed with tap water, 0.6% ammonia water backed to blue, and rinsed with running water. Eosin-stained cytoplasm: sections were stained with eosin staining solution for 1–3 min. Dehydration and sealing: put the slices into gradient concentration (70–100%) ethanol, and xylene to dehydrate and transparent. Took the slices out of the xylene and dry them slightly. Sealed the slices with neutral gum.
Masson staining steps: Dewaxed the slices to distilled water; Hematoxylin stained for 5~10 min; Differentiated of hydrochloric acid alcohol; Washed with distilled water; Stained in ponceau acid fuchsin solution for 5–8 min; Distilled water washed; 1% molybdophosphoric acid for 1~3 min; Directly poured aniline blue solution staining for 5 min without washing; Quick washed with water, dried in a 60°C oven; Rinsed in xylene and sealed; Finally, collagen fibers were blue, muscle fiber and cellulose were red.
Statistical Analysis
Statistical analysis was performed using the one-way ANOVA test for experiments consisting of more than two groups, and with a two-tailed, unpaired t-test in experiments with two groups. Statistical significance was assessed at *p < 0.01, **p < 0.05, ***p < 0.01. NS stands for no statistical significance.
Results
Basic Properties of SE-LNPs
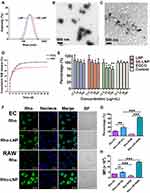
Liposome nanoparticles had been successfully prepared with multiple functions (Figure 1). SE-LNPs were spherical and around 200 nm in diameter under TEM (Figure 2B). The size of LNPs and SE-LNPs were (185.47±0.61) nm and (208.90±3.99) nm respectively (Table S1; Figure 2A). The Zeta potential was almost −20 mV. The polydispersity index of all nanoparticles was less than 0.2. The loading capacity (LC) of SIM in SE-LNPs and encapsulation efficiency (EE) were (43.39±7.6) % and (93.57±1.28) % respectively. The results showed that the loading capacity of EGCG (about 9%) was lower than that of SIM. The morphology of the SE-LNPs was irregular under the condition of hydrogen peroxide (Figure 2C). SE-LNPs had slow-release behavior and released quickly under hydrogen peroxide conditions after 3 days (Figure 2D). After 35 days of release, it showed that about 80% of drugs were released under the condition of hydrogen peroxide, while 62% were released under the condition of PBS. Its encapsulation efficiency and drug loading were very high, which showed that the drug was effectively encapsulated. It met the basic requirements of nano preparations.
SE-LNPs Had Little Cytotoxicity to Macrophages
The results showed that SE-LNPs had good biocompatibility and safety, and there was no significant difference between the proliferation level of cells and untreated cells. (Figure 2E) The results also showed that high concentrations of EGCG were cytotoxic, so the encapsulation of EGCG by LNPs also helped to reduce its toxicity. And there was no significant difference in toxicity between LNPs and SE-LNPs of different concentrations (12.5, 25, 50, 100 μg/mL).SE-LNPs had little cytotoxicity to macrophages and were safe at cell levels.
Rho-LNPs Were More Likely to Be Phagocytized by Macrophages
The results showed that Rho-LNPs were more phagocytic than free Rho. Free Rho had little selectivity for EC and RAW, and even phagocytosis was more in EC cells. The results showed that the Rho-LNPs phagocytic percentage and average fluorescence intensity of nanoparticles in macrophages were higher than those in endothelial cells (Figure 2F–H). It indicated that Rho-LNPs had certain selectivity, which was more conducive to being phagocytized by diseased macrophages to achieve the purpose of targeting. On the one hand, this selectivity was that macrophages were easy to swallow foreign nanoparticles; On the one hand, the passive targeting of nanoparticles was easy to accumulate in inflammatory macrophages.
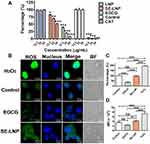
Concentration-Dependent Clearance of ROS by SE-LNPs
The results showed that with the increase in the concentration of EGCG and SE-LNPs, the scavenging capacity of H2O2 in vitro gradually increased (Figure 3A). LNP had no scavenging capacity of H2O2. The positive control catalase had a strong scavenging effect on H2O2, followed by EGCG, while SE-LNP had a mild scavenging effect, which could scavenge ROS without reducing too much. SE-LNPs would degrade under H2O2 conditions because the EGCG in the SE-LNPs would react with H2O2. The amount of EGCG released from the SE-LNPs would be the key component for removing H2O2.
SE-LNPs Removed ROS from Cells
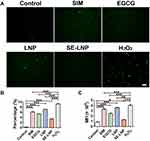
The control group without any treatment also produced a certain amount of ROS, indicating that a certain level of ROS is necessary for cells to maintain normal function and plays an important role in maintaining physiological balance. After preincubation with nanoparticles, intracellular ROS decreased (Figure 3B–D).
The level of ROS in EGCG and SE-LNPs groups decreased significantly, and the inhibition effect of high-concentration nanoparticles on ROS was stronger than that of low-concentration nanoparticles. When the concentration of SE-LNPs reaches 25 μg/mL, the level of ROS produced by cells was similar to that of normal cells (Figure 3B–D). By comparing the antioxidant effect of EGCG with that of SE-LNP, we found that EGCG had a stronger antioxidant effect, so the slow release of EGCG in SE-LNP could appropriately reduce ROS. The results showed that SE-LNP had a significant antioxidant effect and could significantly eliminate ROS compared with the H2O2 group. Its effect after elimination had no statistical difference from that of the negative control group, indicating that it reduced ROS to a normal level rather than excessively reducing ROS.
SE-LNPs Inhibited Apoptosis Caused by ROS
Early apoptosis and necrosis of cells will be cleared by local phagocytes.19 With the progress of AS, the phagocytic system is oversaturated, which in turn induces macrophages and monocytes to form foam cells.20 Apoptosis and necrosis eventually form the necrotic core.21,22 The regulation of apoptosis and necrosis of cells is very important for the process of AS. Our SE-LNPs have been proven to have the effect of inhibiting H2O2-induced apoptosis at the cell level, providing an experimental basis for subsequent animal experiments (Figure 4). SIM and EGCG showed a certain anti-apoptotic effect, but no significant compared with SE-LNPs. And the cell condition after anti-apoptosis of SE-LNPs was the closest to that of the negative control group, which showed that it recovered to a level close to normal.
SE-LNPs Induced the Polarization of RAW264.7 Towards M2
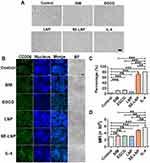
IL-4 significantly induced RAW264.7 to M2. In terms of morphology, IL-4 lengthened the morphology of macrophages and SE-LNPs lengthened the morphology of cells as well. There was little difference between other groups (Figure 5A). The M2 polarization effect of IL-4 was the best, followed by EGCG and SE-LNPs (Figure 5B). Under the same conditions, SE-LNPs promoted more M2 cells than SIM and EGCG. The quantitative results showed that the expression level of M2-induced marker CD206 of IL-4 and SE-LNPs was the strongest (Figure 5C and D).
SE-LNPs Inhibited the Production of Inflammatory Cytokines
We tested the content of related inflammatory cytokines in the supernatant of cell culture to prove SE-LNP’s inhibitory effect on TNF-α, IL-1β, and IL-6 (Figure S1). MCP-1 had little difference in the in vitro experiments of each group. There was only macrophage in vitro and there was no need for monocyte recruitment, so MCP secretion was less. SE-LNPs inhibited the production of some inflammatory cytokines.
Oil Red O Staining Results
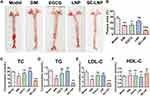
From the Oil red O staining of the whole aorta, the aortic plaque area of ApoE−/− mice fed with high fat for 8 weeks was about 20% of the total area, and the plaque area was large, which was likely to cause acute cardiovascular events. After treatment with EGCG and blank nanoparticles LNPs, the aortic plaque area was almost not reduced. Plaque decreased slightly under the action of free drugs-SIM. After treatment with SE-LNPs, the plaque area reduction effect was better than that of the other groups and had a significant difference (Figure 6A and B).
Hypolipidemia Effect of SE-LNPs on Atherosclerosis
Plasma concentrations of TC, LDL-C, and TG in the SE-LNPs group were significantly different among the treatment groups (Figure 6C–E). SIM could also reduce lipids, but the effect was not as significant as SE-LNPs. The results showed that nanoparticles enhanced the effect of drugs. There was no significant difference in EGCG and LNP groups compared with the control group. The level of HDL-C was increased a little but not significantly (Figure 6F).
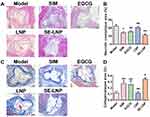
HE and Masson Staining Results of the Aortic Root
We performed HE staining on the aortic root, and the results were consistent with those of Oil red O staining. The necrotic core in the plaque area of the aortic root in each treatment group was lower than that in the model group, while that in the SE-LNPs group was significantly lower, which was significantly different from that of free drugs (Figure 7A and B). It could be observed that there were a large number of fat plaques, in other words, a large number of foam cells. In the control group and LNP group, there were a lot of foam cells in the focus. While the SIM group and EGCG group had a certain reduction effect on foam cells. SE-LNP greatly reduced foam cells, and the vascular morphology was very similar to that of normal blood vessels, which showed that it had the effect of inhibiting the formation of foam cells.
The collagen in the plaque area of the aortic root in the SE-LNPs treatment group was significantly higher than that in the model group (Figure 7C and D). SIM and EGCG had a certain tendency to increase collagen. But there was no statistical difference. The results showed that SE-LNPs had a good recovery effect on atherosclerotic plaque.
Discussion
Moderate concentration of ROS plays an important role in physiological conditions. However, when the existing antioxidant defense system is exceeded, excessive or sustained production of ROS will lead to oxidative stress.23 Animal studies have provided convincing evidence to prove the role of vascular oxidative stress in atherosclerosis. Cardiovascular risk factors, such as hypercholesterolemia, hypertension, diabetes, and smoking, can increase ROS production.24 Vascular oxidative stress promotes the oxidative modification of lipoproteins and phospholipids, endothelial cell activation, and macrophage infiltration/activation. The focus developed in the vascular area (arch, branch, and bifurcation) with blood flow disorder;25 These sites are associated with increased oxidative stress. Therefore, the prevention of vascular oxidative stress is also a reasonable treatment. The results showed that SE-LNPs could clear ROS to the normal level, which was conducive to the restoration of its microenvironment to normal and was critical for the treatment of atherosclerosis.
In preparation, DSPC was the main raw material, and we also added a small amount of 2-Distearoyl-sn-Glycero-3-Phosphoethanolamine conjugated polyethylene glycol (DSPE-PEG) to increase the solubility of EGCG. DSPE-PEG is a kind of functional polyethylene glycol preparation, which is hydrophilic and hydrophobic in combination with phospholipids and polyethylene glycol. Polyethylene glycol phospholipid liposomes form high-quality materials and can be used for drug delivery, gene transfection, and vaccine delivery. PEGylated phospholipids can significantly improve blood circulation time and stabilize drug encapsulation. These materials can also be used for targeted drug delivery by modifying the ligands with target surfaces, such as antibodies, peptides, and liposomes. EGCG is water soluble. So we added the DSPE-PEG in the preparation to improve the EGCG drug loading and encapsulation efficiency. Maybe the thin film method would be not suitable for loading EGCG. In future experiments, we will try different methods to find more appropriate ways to improve the encapsulation efficiency of water-soluble drugs.
The differential subtypes of macrophages in atherosclerotic plaques and their different functional roles were based upon microenvironments such as lipid, intraplaque hemorrhage, and plaque regression.26 M0 macrophages can induce M2 substitutes through the stimulation of IL-4. M2 macrophages express high levels of CD206 and produce anti-inflammatory cytokines. M1, M2, and M4 macrophages were found in atherosclerotic plaque. In plaque, macrophages are strongly influenced not only by cytokines and chemokines but also by bioactive lipids such as cholesterol and oxidized phospholipids. The macrophage phenotype is plastic, so it can be transformed into pro-inflammatory (ie, pro-atherosclerotic) and anti-inflammatory (ie, atherosclerotic protection).27 Therefore, the SE-LNPs provided ROS scavenging function, created a relatively normal environment, inhibited cell apoptosis, had anti-inflammatory and anti-oxidation effects, and were more helpful to transform inflammatory M1 cells into anti-inflammatory M2 cells, to better cooperate with M2 to fight atherosclerosis.
A lot of experimental and clinical evidence indicates that atherosclerosis is a chronic inflammatory disease. Inflammation is the core of all stages of atherosclerosis. It is related to the formation of early fat stripes. When endothelial cells are activated and express chemokines and adhesion molecules, monocytes/lymphocytes recruit and infiltrate into the endothelium.28 The adaptive immune system in atherosclerosis can be pro- or anti-inflammatory and thus pro- or anti-atherogenic.29 TNF-α is associated with an increased risk of cardiovascular death.30 TNF levels are used to predict the severity of peripheral arterial disease and are also associated with the burden of atherosclerosis.31 A large amount of evidence shows that IL-1 cytokines are related to the pathogenesis of cardiovascular disease.32 The future may include targeted inhibitors that block IL-1 subtypes for a wide range of cardiovascular diseases. Elevated IL-6 levels are associated with an increased risk of myocardial infarction in the future. Some research data support the role of cytokine-mediated inflammation in the early stage of atherosclerosis.33 When cytokines activate MMP and vascular cells in macrophages and promote cell apoptosis, leading to easier rupture or corrosion of the balance between pro-inflammatory and anti-inflammatory, cytokines have become the main determinant of plaque. So the treatment of inflammation in atherosclerosis with SE-LNPs was of great significance to the current and future treatment methods. New targets might appear in the regulation of inflammation levels.
Simvastatin treatment could significantly decrease lipid accumulation.34 Simvastatin is a long-standing HMG CoA reductase inhibitor; LDL-C is decreased, while TC and ApoB are decreased, and HDL-C is moderately increased.35 Jun-bo Ge groups proved that simvastatin inhibits vascular inflammation and atherosclerosis in ApoE−/− mice, which may be mediated by down-regulating the hmgb1-rage axis.36 However, this kind of statin is not the best. Now there are other statins like rosuvastatin with more obvious efficacy,37 but they are expensive. However, the SE-LNPs could increase their efficacy, that is, we continued to use this old drug at a low cost when the efficacy could be guaranteed.
The formation of foam cells and the accumulation of vascular endothelium are the markers of atherosclerosis. Targeting the formation of foam cells may be a promising method for the treatment and prevention of atherosclerosis. The formation of foam cells depends on the balance effects of three major related biological processes, including lipid uptake, cholesterol esterification, and cholesterol efflux.38 Our preparation could also inhibit the formation of foam cells as shown in HE staining results, and the liposomes were engulfed in large quantities by macrophages, thus inhibiting other lipids’ uptake. At the same time, it may also promote the hydrolysis of cholesterol esters and the outflow of cholesterol, showing the ability to resist atherosclerosis. The further development of liposome nanoparticles is needed to study.
The level of anti-oxidation, anti-apoptosis as well as M2 polarization by SE-LNPs in the atherosclerotic plaques were evaluated. The antioxidant capacity was medium because its antioxidant capacity was not as strong as EGCG, but appropriate antioxidant capacity could help it maintain ROS at the normal physiological level and maintain normal signal exchange, rather than causing too little ROS to form reduction stress. The anti-apoptosis ability was relatively strong. Compared with the positive control of hydrogen peroxide, it significantly reduced the apoptotic cells, which was conducive to the normal work of cells and reduced the damage to the oxidative microenvironment. The induction effect of M2 was moderate, without a significant effect of IL-4. However, compared with other groups, it had a great induction effect, that is to say, it improved the bioavailability of drugs, and appropriate M2 induction was helpful for the immune repair of diseases, without excessive fibrosis. In a word, all indicators have been improved to a certain extent, with a significant anti-atherosclerosis effect.
Conclusion
A liposome nanoparticle delivery system (SE-LNPs) based on ROS responsiveness, anti-apoptosis, anti-inflammation, and M2 polarization was constructed for the first time, loaded with simvastatin and EGCG for the prevention and treatment of atherosclerosis. The synthetic method of ROS-responsive multifunctional nano drugs is simple and mature and has good biosafety. It can play a good anti-atherosclerotic effect. The results of in vivo and in vitro experiments show that SE-LNPs were superior to free drugs and LNPs. At the same time, it could ameliorate the environment. It could carry out anti-oxidative stress, anti-apoptosis, and anti-inflammatory effects, and achieve the purpose of single-system multi-functional synergistic treatment, to provide new research ideas for the selective treatment of atherosclerosis.
Abbreviations
SIM, simvastatin; DSPC, distearyl phosphatidylcholine; EGCG, epigallocatechin gallate; SE-LNPs, simvastatin and EGCG liposome nanoparticles; ROS, reactive oxygen species; PBS, phosphate-buffered saline; Rho, Rhodamine-DPPE; TEM, transmission electron microscope; H2O2, hydrogen peroxide; HPLC, high-performance liquid chromatography; RAW264.7, mice macrophages; HUVEC, human umbilical vein endothelial cells; HE, hematoxylin-eosin; TC, total cholesterol; TG, triglyceride; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; NS, no statistical significance.
Acknowledgments
Financial support from National Natural Science Foundation of China (82170274); Guangdong Basic and Applied Basic Research Foundation (2022A1515011747); Guangzhou Key Research & Development Program (202206010065); Medical Scientific Research Foundation of Guangdong Province, China (A2022547).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Marchio P, Guerra-Ojeda S, Vila JM, Aldasoro M, Victor VM, Mauricio MD. Targeting early atherosclerosis: a focus on oxidative stress and inflammation. Oxid Med Cell Longev. 2019;2019:8563845. doi:10.1155/2019/8563845
2. Zheng Y, Lim EJ, Wang L, Smart EJ, Toborek M, Hennig B. Role of caveolin-1 in EGCG-mediated protection against linoleic-acid-induced endothelial cell activation. J Nutr Biochem. 2009;20(3):202–209. doi:10.1016/j.jnutbio.2008.02.004
3. Eng QY, Thanikachalam PV, Ramamurthy S. Molecular understanding of Epigallocatechin gallate (EGCG) in cardiovascular and metabolic diseases. J Ethnopharmacol. 2018;210:296–310. doi:10.1016/j.jep.2017.08.035
4. Duan J, Chen Z, Liang X, et al. Construction and application of therapeutic metal-polyphenol capsule for peripheral artery disease. Biomaterials. 2020;255:120199. doi:10.1016/j.biomaterials.2020.120199
5. Basu A, Lucas EA. Mechanisms and effects of green tea on cardiovascular health. Nutr Rev. 2007;65(8 Pt 1):361–375. doi:10.1111/j.1753-4887.2007.tb00314.x
6. Shapouri‐Moghaddam A, Mohammadian S, Vazini H, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425–6440. doi:10.1002/jcp.26429
7. Colin S, Chinetti-Gbaguidi G, Staels B. Macrophage phenotypes in atherosclerosis. Immunol Rev. 2014;262(1):153–166. doi:10.1111/imr.12218
8. Cai F, Liu S, Lei Y, et al. Epigallocatechin-3 gallate regulates macrophage subtypes and immunometabolism to ameliorate experimental autoimmune encephalomyelitis. Cell Immunol. 2021;368:104421. doi:10.1016/j.cellimm.2021.104421
9. Granja A, Frias I, Neves AR, Pinheiro M, Reis S. Therapeutic potential of epigallocatechin gallate nanodelivery systems. Biomed Res Int. 2017;2017:5813793. doi:10.1155/2017/5813793
10. Zhang J, Nie S, Zu Y, et al. Anti-atherogenic effects of CD36-targeted epigallocatechin gallate-loaded nanoparticles. J Control Release. 2019;303:263–273. doi:10.1016/j.jconrel.2019.04.018
11. Wang S, Su R, Nie S, et al. Application of nanotechnology in improving bioavailability and bioactivity of diet-derived phytochemicals. J Nutr Biochem. 2014;25(4):363–376. doi:10.1016/j.jnutbio.2013.10.002
12. Singh AP, Biswas A, Shukla A, Maiti P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct Target Ther. 2019;4:33. doi:10.1038/s41392-019-0068-3
13. Borborema SE, Schwendener RA, Osso JA, de Andrade HF, Do Nascimento N. Uptake and antileishmanial activity of meglumine antimoniate-containing liposomes in Leishmania (Leishmania) major-infected macrophages. Int J Antimicrob Agents. 2011;38(4):341–347. doi:10.1016/j.ijantimicag.2011.05.012
14. Malik DJ, Sokolov IJ, Vinner GK, et al. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv Colloid Interface Sci. 2017;249:100–133. doi:10.1016/j.cis.2017.05.014
15. Wang N, Chen M, Wang T. Liposomes used as a vaccine adjuvant-delivery system: from basics to clinical immunization. J Control Release. 2019;303:130–150. doi:10.1016/j.jconrel.2019.04.025
16. Darwitan A, Wong YS, Nguyen LTH, et al. Liposomal nanotherapy for treatment of atherosclerosis. Adv Healthcare Mater. 2020;9(14):e2000465. doi:10.1002/adhm.202000465
17. Kiaie N, Gorabi AM, Penson PE, et al. A new approach to the diagnosis and treatment of atherosclerosis: the era of the liposome. Drug Discov Today. 2020;25(1):58–72. doi:10.1016/j.drudis.2019.09.005
18. Al-Amin MD, Bellato F, Mastrotto F, et al. Dexamethasone loaded liposomes by thin-film hydration and microfluidic procedures: formulation challenges. Int J Mol Sci. 2020;21(5):1611. doi:10.3390/ijms21051611
19. Tabas I. Apoptosis and efferocytosis in mouse models of atherosclerosis. Curr Drug Targets. 2007;8(12):1288–1296. doi:10.2174/138945007783220623
20. Zernecke A, Winkels H, Cochain C, et al. Meta-analysis of leukocyte diversity in atherosclerotic mouse aortas. Circ Res. 2020;127(3):402–426. doi:10.1161/CIRCRESAHA.120.316903
21. Nakagawa K, Tanaka M, Hahm TH, et al. Accumulation of plasma-derived lipids in the lipid core and necrotic core of human atheroma: imaging mass spectrometry and histopathological analyses. Arterioscler Thromb Vasc Biol. 2021;41(11):e498–e511. doi:10.1161/ATVBAHA.121.316154
22. Bäck M, Yurdagul A, Tabas I, Öörni K, Kovanen PT. Inflammation and its resolution in atherosclerosis: mediators and therapeutic opportunities. Nat Rev Cardiol. 2019;16(7):389–406. doi:10.1038/s41569-019-0169-2
23. Kattoor AJ, Pothineni NVK, Palagiri D, Mehta JL. Oxidative stress in atherosclerosis. Curr Atheroscler Rep. 2017;19(11):42. doi:10.1007/s11883-017-0678-6
24. Förstermann U, Xia N, Li H. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ Res. 2017;120(4):713–735. doi:10.1161/CIRCRESAHA.116.309326
25. DeCarlo C, Latz CA, Boitano LT, et al. Natural history of penetrating atherosclerotic ulcers in aortic branch vessels. J Vasc Surg. 2021;74(6):1904–1909. doi:10.1016/j.jvs.2021.06.035
26. Jinnouchi H, Guo L, Sakamoto A, et al. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell Mol Life Sci. 2020;77(10):1919–1932. doi:10.1007/s00018-019-03371-3
27. Chistiakov DA, Bobryshev YV, Orekhov AN. Changes in transcriptome of macrophages in atherosclerosis. J Cell Mol Med. 2015;19(6):1163–1173. doi:10.1111/jcmm.12591
28. Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86(2):515–581. doi:10.1152/physrev.00024.2005
29. Wolf D, Ley K. Immunity and inflammation in atherosclerosis. Circ Res. 2019;124(2):315–327. doi:10.1161/CIRCRESAHA.118.313591
30. Chen AQ, Fang Z, Chen XL, et al. Microglia-derived TNF-α mediates endothelial necroptosis aggravating blood brain-barrier disruption after ischemic stroke. Cell Death Dis. 2019;10(7):487. doi:10.1038/s41419-019-1716-9
31. Bruunsgaard H, Andersen-Ranberg K, Jeune B, Pedersen AN, Skinhøj P, Pedersen BK. A high plasma concentration of TNF-alpha is associated with dementia in centenarians. J Gerontol a Biol Sci Med Sci. 1999;54(7):M357–M364. doi:10.1093/gerona/54.7.M357
32. Abbate A, Toldo S, Marchetti C, Kron J, Van Tassell BW, Dinarello CA. Interleukin-1 and the inflammasome as therapeutic targets in cardiovascular disease. Circ Res. 2020;126(9):1260–1280. doi:10.1161/CIRCRESAHA.120.315937
33. Ridker PM, Rifai N, Stampfer MJ, Hennekens CH. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101(15):1767–1772. doi:10.1161/01.CIR.101.15.1767
34. Wang L, Yao X, Li Q, Sun S. Effect of simvastatin on lipid accumulation and the expression of CXCL16 and nephrin in podocyte induced by oxidized LDL. J Invest Surg. 2018;31(2):69–74. doi:10.1080/08941939.2016.1278057
35. Pedersen TR, Tobert JA. Simvastatin: a review. Expert Opin Pharmacother. 2004;5(12):2583–2596. doi:10.1517/14656566.5.12.2583
36. Liu M, Yu Y, Jiang H, et al. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(-/-) mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol Sin. 2013;34(6):830–836. doi:10.1038/aps.2013.8
37. Karlson BW, Palmer MK, Nicholls SJ, Lundman P, Barter PJ. Doses of rosuvastatin, atorvastatin and simvastatin that induce equal reductions in LDL-C and non-HDL-C: results from the VOYAGER meta-analysis. Eur J Prev Cardiol. 2016;23(7):744–747. doi:10.1177/2047487315598710
38. Wang D, Yang Y, Lei Y, et al. Targeting foam cell formation in atherosclerosis: therapeutic potential of natural products. Pharmacol Rev. 2019;71(4):596–670. doi:10.1124/pr.118.017178