An Overview of Drug Delivery Nanosystems for Sepsis-Related Liver Injury Treatment
Introduction
Sepsis is a life-threatening organ dysfunction resulting from a dysregulated response to infection with high morbidity and mortality.1 There have been more than 48.9 million new sepsis patients worldwide in each of the past three years, and the number of deaths caused by multiorgan failure is as high as 11 million, accounting for 19.7% of global deaths, which poses a great threat to human health worldwide.2 The liver is a substantial organ in the human body and is involved in physiological processes such as immunity, metabolism, and detoxification. The liver is one of the most susceptible organs to sepsis. Sepsis-related liver injury (SRLI) refers to liver damage caused directly or indirectly by sepsis, resulting abnormalities in biochemical tests and liver insufficiency, or even liver failure.3
Liver injury occurs at any stage of sepsis, especially in patients with underlying liver disease. Early detection and intervention can improve patient survival rates to some extent.4,5 Drug treatment of SRLI is mainly based on conventional empirical methods, including antibiotic, antioxidant, antibacterial, and anti-platelet aggregation treatments.6–8 These therapies have three main shortcomings. First, many drugs lack either specific targeting to precisely treat damaged tissues or high bioavailability. Small-molecule drugs are rapidly cleared in vivo, which seriously affects drug efficacy. For example, during the development of sepsis, cardiac excretion and intrahepatic blood perfusion are increased and reduced, respectively, resulting in impaired hepatic circulatory metabolism, coagulation dysfunction and microthrombosis.9 The small-molecule heparin is commonly used clinically to treat platelet aggregation, but its use is limited by a short half-life and difficulty in controlling the dose.10,11 Second, antibiotics are the standard of clinical care for sepsis; however, the drug resistance and hepatorenal toxicity they cause cannot be ignored, and 70–80% of clinical deaths from sepsis are associated with persistent infection.12,13 For example, in common neonatal infections. E. coli is resistant to β-lactam antibiotics and aminoglycosides, Group B streptococci are resistant to penicillin, and Listeria monocytogenes is resistant to cephalosporins and vancomycin;14 multiple drug resistance poses a great burden to the treatment of neonatal diseases. Third, the single-drug conventional model is ineffective due to the interaction of the enterohepatic axis in liver injury during sepsis. Elevated levels of inflammatory factors and oxygen free radicals in sepsis patients impair hepatic clearance and disrupt the intestinal flora when endotoxins and bacteria enter the liver through the damaged intestinal mucosa via the portal vein or impair liver function and cause inflammatory storms with increased disruption of intestinal microbial composition and diversity;15,16 the complex pathophysiology involving multichannel cytokine storms requires a multipronged approach. The emergence of nanotechnology has created a new avenue to overcome the difficulties of severe drug reactions, drug resistance and overly homogeneous treatment modalities. In recent years, researchers have designed a variety of delivery nanosystems including liposomes,17,18 solid liposomal nanoparticles,19 polymeric micelles,20,21 and extracellular vesicles,22,23 and have tried their best to solve the problems in the treatment of liver injury related to sepsis.
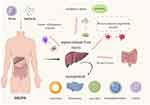
Drug delivery nanosystems have great potential for the accurate treatment of sepsis-related liver injury, mainly due to their physical (size, charge, shape) and chemical properties. For example, different organs can differentially uptake particles with sizes; microsphere (MS) nanoparticles in the 0.5 μm to 5 μm particle size range exhibit ideal lung targeting,24 while nanoparticles larger than 100 nm can passively target hepatic sinusoidal endothelial cells and Kupffer cells (KC), and nanoparticles smaller than 100 nm generally passively target hepatocytes and hepatic stellate cells (HSCs).25 Kupffer cell is the specialized type of macrophages within the hepatic tissue. Under the pathological conditions of sepsis, Kupffer cells can be activated by endotoxin and tumor necrosis factor (TNF) and release inflammatory mediators such as transforming growth factor (TGF), interleukin-6 (IL-6) and oxygen free radicals, which accelerate the process of liver injury. Additionally, the accumulation of spherical mesoporous silica NPs (Nanoparticles) in the liver is higher than that of rod-shaped mesoporous silica NPs. NPs with positively charged surfaces increase cellular interference by adjusting their pH to disrupt the endosomal system, thus targeting hepatocytes.26 In addition, other types of nanoparticles, such as antimicrobial peptides (AMPs),27,28 contain anti-intercellular adhesion molecule-1 (ICAM-1)29 and polymers30 and are promising for improving pharmacokinetics, drug delivery stability and bioavailability, as these agents synergistically promote the use of drug delivery nanosystems for the specific treatment of sepsis-related liver injury. Therefore, in this review (Figure 1), we reviewed drug delivery nanosystems designed to treat SRLI according to different pathogeneses. Furthermore, we also discussed the key issues currently facing the field, including immune system rejection, single treatment modalities, and outlooks for the future.
 |
Figure 1 Pathogenesis of sepsis-related liver injury and drug delivery nanosystems. |
Drug Delivery Nanosystems for the Treatment of Sepsis-Related Liver Injury
The Hepatic Inflammatory Response
When the body is invaded by exogenous pathogens and other agents, the host immune system is activated, and polymorphonuclear neutrophils (PMNs) accumulate to clear pathogens after crossing the vascular barrier; these cells are regulated by apoptosis to maintain constant numbers and homeostasis. However, during sepsis, large amounts of inflammatory factors are released in vivo, and neutrophils are prone to overactivation and uncontrolled infiltration, causing an inflammatory storm, which leads to the development of sepsis.31–33, Zhang et al34 prepared doxorubicin-hydrazone bovine serum albumin nanoparticles (DOX-hyd-BSA NPs) after linking doxorubicin (DOX) to bovine serum albumin (BSA) via pH-sensitive bonding to target proinflammatory neutrophils and evaluated their therapeutic potential in sepsis-induced liver injury and inflammation. Cellular uptake assays revealed that DOX-hyd-BSA NPs were efficiently taken up by differentiated HL-60 cells but not by T cells, monocytes or natural killer (NK) cells. In a mouse model of LPS-induced sepsis, blood neutrophils were collected after intraperitoneal injection of DOX-hyd-BSA NPs, and anti-mouse LY-6G antibody staining revealed that neutrophils were required for NP uptake. In addition, compared to those in the control group, mice in the DOX-hyd-BSA NP group showed significantly improved inflammation with a 72 h survival rate of 70%, and their neutrophil counts and cytokine levels returned to healthy levels. In addition, immunohistochemical analysis showed that the organ toxicity of DOX-hyd-BSA NPs was closely related to the DOX content, and there was no significant overall damage to liver tissue or function. This finding suggested that DOX-hyd-BSA NPs inhibited neutrophil transit and inflammatory responses without hepatotoxicity and were a promising anti-inflammatory agent for liver injury in sepsis. The mechanism is described in Figure 2.
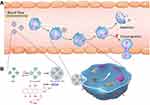 |
Figure 2 Mechanism of DOX-hyd-BSA NPs targeting pro-inflammatory neutrophils to induce their apoptosis for the treatment of septic inflammation. (A) The therapeutic mechanism of DOX-hyd-BSA NPs; (B) The preparation route of DOX-conjugated BSA NPs. Notes: Reprinted from Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5(11):eaax7964. https://creativecommons.org/licenses/by-nc/4.0/.34 |
Cellular autophagy is a natural defense mechanism against pathogens. Cells are induced to produce autophagosomes to deliver pathogens or cellular metabolic waste for degradation and reduce the stress response in vivo.35,36 Sepsis triggers cellular autophagy in several organs, including the liver, and regulates inflammatory vesicles in macrophages to protect the liver from damage.37–39 Previous studies40–42 have demonstrated that superparamagnetic iron oxide nanoparticles (SPIONs) have low cytotoxicity and good biocompatibility, are suitable for studying the migratory behavior and biodistribution of dendritic cells in vivo, and have diagnostic and therapeutic value as inducers of macrophage activation. To better understand the mechanism by which SPIONs regulate inflammation and macrophage autophagy in SRLI, Xu et al43 prepared SPIONs by wrapping a modified polymer (polyglucose sorbitol carboxymethyl ether) around the outer layer of iron oxide and characterized their morphology and magnetic properties. In a mouse model of LPS-induced liver injury in sepsis, SPIONs ameliorated the level of inflammation in sepsis, reduced the infiltration of inflammatory cells in the liver and increased interleukin-10 (IL-10) levels by activating autophagy in hepatic macrophages (macrophages are the most prominent cell population expressing IL-10 in the liver). Furthermore, SPIONs decreased ALT and AST levels by regulating the caveolin-1 (Cav1) and Notch1/HES1 signaling pathways in macrophages. Therefore, it can be concluded that SPIONs activate macrophage autophagy to regulate liver hepatocytes and are a potential therapeutic agent for septic liver injury.
With pathogen invasion and the overactivation of the innate immune response, sepsis-associated inflammation escalates, causing irreversible damage to the liver and eventually leading to multiorgan failure and even death.44,45 Most current single drug nanocarriers of antibiotics act mainly on the bacteria themselves and do not target the tissues that are already inflamed,46–48 so new carriers are needed that can deliver multiple drugs simultaneously and specifically target the damaged tissues to reduce pathogen transmission and the inflammatory response in vivo.49–51 It has been found that a specific microenvironment is formed in infected tissues, and the main features are low pH,52 bacterial enzymes53 and activated blood vessels.54 Based on the results of this infectious microenvironment (IME) study, Zhang et al55 designed a copolymer carrier with a certain pH value and sensitivity to bacterial enzymes (Figure 3) and loaded it with antibiotics (ciprofloxacin) and anti-inflammatory agents (2-[(aminocarbonyl)amino]-5-(4-fluorophenyl)-3-thiophenecarboxamide), after which the nanoparticles were coated with intercellular adhesion molecule-1 (ICAM-1) antibodies targeting the IME. The results showed that when the antibody-coated nanoparticles came in contact with the vascular system of the infected liver tissue, a reduction in pH and enzymatic changes occurred to achieve drug release,56–58 indicating that targeting inflamed liver tissue was valuable for the treatment of sepsis-associated liver injury.
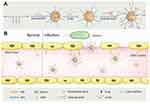 |
Figure 3 Scheme for designing IME responsive and biofunctional nanoparticles (NPs) and for targeted delivery of nanotherapeutics at the site of infection. (A) The designing strategy of intercellular adhesion molecule-1 antibodies-modified multifunctional NPs; (B) The prepared NPs showed a preferential accumulation at a site of infection by interaction with intercellular adhesion molecule-1. Notes: Reprinted with permission from John Wiley and Sons. Zhang CY, Gao J, Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30(43):e1803618. © 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.55 |
Hepatic Oxidative Stress
Increased oxidative stress during sepsis is another mechanism leading to the development of liver injury. Oxygen radicals can trigger lipid peroxidation, producing large amounts of reactive oxygen (ROS) and reactive nitrogen species (RNS), causing severe damage to mitochondria in hepatocytes and inducing apoptosis or necrosis in hepatocytes, eventually leading to liver injury or even failure.59–61 Therefore, antioxidant therapy can be one of the strategies for the treatment of SRLI. Melatonin (Mel) is a multifunctional hormone with strong antioxidant effects, and some studies have shown that systemic administration of free Mel could reduce sepsis-induced tissue damage.62–64 Melatonin is currently mainly used in oral dosage form, but due to its short half-life (t1/2<30 min) and low in vivo absorption, thus limiting its clinical use.65 Chen et al66 prepared methoxypolyethylene glycol-b-poly(propylene sulfide) (PEG-b-PPS) by adding polyvinyl alcohol (PVA) to a solution containing Mel solution after ultrasonic emulsification to obtain Mel polymer nanoparticles (mPEG-b-PPS-NPs) with ROS-responsive effects and determined the sensitivity of the nanoparticles to oxidative stress in the septic liver. First, hydrogen peroxide (H2O2) was used to simulate oxidative stress, and measurements showed that in the absence of H2O2, NPs were morphologically intact, but when exposed to 10 mM H2O2, NPs appeared to swell and disintegrate, indicating that mPEG-b-PPS-NPs were highly sensitive to oxidative stress. Liver serum malondialdehyde was then measured in mice with LPS-induced sepsis to detect oxidation. Malondialdehyde (MDA), which are the major products of unsaturated fatty acids, are markers of oxidative stress, and excessive levels trigger lipid peroxidation.67,68 The mPEG-b-PPS-NP group had significantly reduced MDA levels in the liver compared with the free Mel alone group. The plasma ALT and AST activities of mice were inhibited. Finally, the degree of proinflammatory neutrophil infiltration was measured in septic mice with liver injury, and the results showed that mice with infectious shock had increased inflammatory factor activity, whereas mPEG-b-PPS-NPs accelerated proinflammatory neutrophil apoptosis and significantly eliminated sepsis-induced liver inflammation compared with a slightly nonsignificant effect of the same dose of free Mel. It was evident that Mel-loaded mPEG-b-PPS-NPs had the potential to treat liver injury in sepsis.
Oxygen radicals and their triggered peroxidation reactions cause severe mitochondrial damage in hepatocytes, affecting membrane protein function, impairing hepatic energy metabolism and detoxification, and exacerbating the extent of liver injury.69,70 To better target mitochondria, Yu et al71 synthesized organic polymer (mPEG-TK-PLGA) and atorvastatin-loaded nanoparticles (PTP-TCeria NPs) as the core part of the structure and then wrapped the nanoparticles with CeO2 (TCeria) modified with triphenylphosphine in the outer layer to prepare an atorvastatin-loaded nanodelivery system (Atv/PTP-TCeria NPs). H2O2-stimulated human umbilical vein endothelial cells (HUVECs) were used as a cell model of oxidative stress injury. After injection of the NPs and incubation for 24 h, the Atv/PTP-TCeria NP group showed lower cell mortality and significantly higher superoxide dismutase (SOD) levels than the other groups, indicating better antioxidant effects. PTP-TCeria NPs could inhibit H2O2-induced apoptosis and had a strong ability to scavenge ROS. Immunohistochemical analysis of mouse organs showed that the Atv/PTP-TCeria NP treatment group exhibited normal structures in the kidney and liver, and the levels of liver function-related indicators (aspartate aminotransferase (AST) and alanine aminotransferase (ALT)) were mostly unaffected compared with those in the AKI group. In contrast, there was extensive congestion and necrosis in the liver and kidney tissues of mice in the LPS group.
Sepsis-induced apoptosis or necrosis in hepatocytes is the central aspect of liver injury during sepsis. When bacteria, endotoxin and inflammatory factors act on hepatocytes, they activate the liver’s apoptotic mechanism to clear the damaged hepatocytes. However, prolonged pathogens and inflammation cause high levels of hepatocyte apoptosis to occur, leading to mitochondrial depletion and the inability to activate caspase-3, resulting in hepatocyte necrosis.72–74 To better protect hepatocytes, Ye et al75 formed a nicotinamide purine dinucleotide (NAD+)-loaded liposome nanoparticle (NAD+-LP-Cap) coated with calcium phosphate (Cap) on the surface to help NAD+ operate better in the blood circulation and provided energy to cells to prevent apoptosis. Moreover, NAD+-LP-Cap improved cellular uptake of NAD+ and reduced mortality in mice with sepsis induced by Pseudomonas aeruginosa after appendiceal ligation and puncture and could be an innovative treatment modality for liver injury in sepsis.
In addition, a summary of drug delivery nanosystems with therapeutic potential for antioxidant effects on liver injury in sepsis can be found in Table 1.
 |
Table 1 Antioxidant Nanodrug Delivery System with Potential to Alleviate Liver Injury in Sepsis |
Hepatic Microcirculatory Coagulation Disorders
Hepatic microcirculatory coagulation disorders are also triggers that exacerbate sepsis-related liver injury. The activation and overexpression of viscous molecules by hepatic sinusoidal endothelial cells during sepsis lead to fibrin deposition and microthrombus formation in the hepatic microvasculature. Microcirculatory impairment reduces vascular blood perfusion, and hepatic ischemia occurs, along with the depletion of platelets and coagulation factors, leading to coagulation dysfunction and eventual bleeding.87,88 The aqueous extract of D. donglingii leaves is a terpenoid that has various biological and pharmacological effects, such as anticancer, antithrombotic and anti-inflammatory effects. However, its clinical application is limited due to its rapid degradation and low bioavailability. For this reason, Wang et al89 prepared solid nanoliposomal particles loaded with aqueous extracts of Dondelia leaf (AERL NPs) through spin filtration and other means and evaluated its therapeutic potential in promoting intrahepatic microcirculation. The in vitro platelet aggregation assay showed that the aqueous extract of D. melanogaster leaves (AERL) could inhibit platelet aggregation induced by thrombin, adenosine diphosphate and platelet activating factor (PAF) with half-inhibitory concentrations of 0.12–1.43 mg/mL and inhibited the release of platelet P-selectin in a concentration-dependent manner. After oral administration of different doses of AERL NPs (5 mg/kg, 25 mg/kg, 50 mg/kg), blood was collected from the rats. The characterization of AERL NPs in plasma showed that the diameters of the NPs increased with decreasing size, which allowed the selection of different concentrations for targeting hepatic sinusoidal endothelial cells and hepatocytes. The intrahepatic thrombus weight of rats was measured by flow cytometry, and the thrombus weights of rats in the AERL NP group were significantly lower than those in the oral NS group at minimum effective dose of 5 mg/kg. Therefore, AERL NPs have ideal antithrombotic properties.
Histones are a group of nuclear proteins with positive ions and are responsible for packaging DNA into chromatin and regulating gene expression. During sepsis, histones are released into the circulation and mediate inflammation, organ damage, and failure by activating toll-like receptors (TLRs) and NLRP3 inflammatory vesicles and induce coagulation by binding to platelets. High histone concentrations are characteristic of severe sepsis and high mortality.90–92 H3 and H4 histones have now been shown to improve survival in mouse models of sepsis.93,94 To better target histones and prolong the duration of nanodrugs, Koide et al44 (Figure 4) prepared poly(ethylene glycol) methacrylate by modified precipitation polymerization and then combined various functional monomers to prepare PRG-modified lightly cross-linked N-isoacrylamide-based hydrogel polymer NPs (PEG-HNPs). In vivo distribution showed that PEG-HNPs accumulated mainly in the liver (~50%) at 3 h after injection in mice and remained in the bloodstream for 24 h, increasing the binding of HNPs to histone targets. Cy histone signals in the lung, kidney and intestine were significantly weaker after PEG-HNP treatment than after cy5-HNP injection alone, indicating that PEG-HNPs can capture histones in the blood and target the liver. Cellular colocalization showed that PEG-HNPs entered the cells through the cell membrane and that the histones they carried were not released into the cytoplasm, nor did they induce toxicity. After tail vein injection of PEG-HNPs (10 mg/kg) in mice, the researchers measured the changes in platelet and found that PEG-HNPs significantly increased platelet counts, prolonged bleeding time, inhibited platelet activation and thrombosis and improved vascular microcirculation.
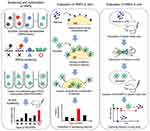 |
Figure 4 Preparation and efficacy assessment of PEG-HNPs for sepsis treatment. Notes: Reprinted from Koide H, Okishima A, Hoshino Y, et al. Synthetic hydrogel nanoparticles for sepsis therapy. Nat Commun. 2021;12(1):5552. https://creativecommons.org/licenses/by/4.0/.44 |
Bacterial Translocation
The “gut-liver” axis plays an important role in the pathogenesis of sepsis. The intestine is the largest reservoir of bacteria in the body, and the liver is the main site of bacterial infection. Patients with sepsis often have an imbalance in intestinal flora, which is accompanied by ischemia and hypoxia in the intestinal mucosa, which leads to the invasion of endotoxin and bacteria into the liver through the damaged intestinal mucosa with portal vein and causes intestinal inflammation and liver injury.95–97 Thus, the choice of drugs needs to satisfy both low hepatotoxicity and good intestinal antimicrobial properties. Acharya et al98 prepared spherical silver nanoparticles (AgNP-sp) and rod-shaped silver nanoparticles (AgNR) and evaluated their antimicrobial activity against various Gram-negative and Gram-positive bacteria by using citric acid thermal reduction and surfactant-free wet chemical methods. Electron microscopic characterization revealed that the addition of citrate ions during the synthesis of colloidal AgNPs allowed the entire particle to be negatively charged, avoiding particle aggregation, and that the two nanoparticles were face cubic center (fcc) structures with particle sizes of 40–50 nm and 20 ± 6 nm, respectively. MIC values were used to study their bactericidal kinetics (105 CFU/ml in primary cells), and the results suggested that these nanoparticles had significant growth inhibitory effects on Gram-negative and Gram-positive bacteria; smaller particle sizes (5–10 nm) were associated with better antibacterial effects, while higher concentrations were associated with better effects. Finally, it was found that the spherical AgNP-sp was able to bind tighter to the intestinal folds due to its large contact area, and its inhibitory and bactericidal activity was more significant than that of the rod-shaped AgNR, which is consistent with the previous report showing the killing of the tested bacteria within 3 h.99
Enterobacteriaceae is one of the most frequent infectious agents associated with sepsis, and carbapenem antimicrobials are preferred as clinical treatments. Meropenem is a common choice for the treatment of Enterobacteriaceae; however, its use is restricted due to its short half-life (approximately 1 hour), risk of liver damage, and susceptibility to resistance.100,101 Memar et al102 prepared meropenem monodisperse silica microspheres (MSNs) with an average diameter of 68.43 nm, which had different shapes (spherical, long rod, short rod, cubic) and negatively charged surfaces. Meropenem synergizes with silica to better inhibit bacterial cell wall synthesis and achieves antibacterial effects. In vivo biodistribution analysis revealed that the MSNs were mostly present in the liver and kidney in Enterobacteriaceae-infected mice. Long rod-shaped MSNs had a high potential to overcome rapid clearance by the reticuloendothelial system and remained in vivo for a long period of time, while spherical MSNs were rapidly cleared in the liver and had few toxic effects. In addition, in vitro bacterial assays showed that MSNs loaded with meropenem showed better clearance of Enterobacteriaceae than free meropenem. Hemolysis assays and cytotoxicity assays showed that MSNs were nontoxic, noncytotoxic and hemocompatible particles. Therefore, MSNs loaded with meropenem served as a potential antimicrobial agent against sepsis liver injury.
In addition to these treatment modalities targeting the “gut-liver” axis, restoring the intestinal flora is also a promising therapeutic strategy that can contribute to the repair of liver injury due to sepsis. Fan et al103 obtained ROS-responsive nanoparticles (GEN-NPs) by coupling the SOD mimetic tempol (TPL) to β-cyclodextrin (β-CD) and then modifying it with 4-(hydroxymethyl)dumb-borate. The nanoparticles had strong colonic targeting and could effectively increase the expression of estrogen receptor β (ER-β), decrease the expression of Caspase-1, increase field permeability, help the intestinal flora “self-heal” and achieve anti-inflammatory effects in the context of a large amount of oxygen free radical secretion.Drug delivery nanosystems that could be used to treat bacterial translocation in SRIL are summarized in Table 2.
 |
Table 2 Summary of Drug Delivery Nanosystems for Bacterial Translocation in SRIL |
Other Drug Delivery Nanosystems
It has been recently shown that mesenchymal stromal cells (MSCs) and extracellular vesicles (EVs) (MSCs secreted) can exert anti-inflammatory effects on the inflammatory environment associated with sepsis.106 However, the low secretion of EVs and complicated isolation procedures limit their widespread use. To address this problem, Park et al107 prepared nanovesicles (NVs) with high similarity to EVs and analyzed the potential of the NVs to treat sepsis-associated inflammation and liver injury. The preparation of NVs was followed by transmission electron microscopy and nanoparticle tracking to analyze the physical properties of the NVs, and the results showed that the NVs had a spherical structure with a diameters 50–150 nm; GO analysis was performed and suggested that the proteins that make up the NVs were involved in inflammatory, gonadotropic and angiogenic pathways. Subsequently, and in vivo sepsis model was established by intraperitoneal injection of E. coli (OMVs, 15 μg) followed by intraperitoneal injection of NVs (2* 109) for 6 h. NVs abrogated the reduction in ocular secretions, decreased body temperature (not entirely to normal levels) and the number of neutrophils and monocytes in the mice and inhibited tumor necrosis factor (TNF-α) and interleukin 6 (IL-6) expression; NIR imaging analysis with Cy7 dye labeling at 6 h revealed that NVs localized to the liver, spleen, and lung and disappeared after 24 h. Western blot analysis showed that NVs ameliorated the inflammatory response in mice induced by E. coli. In vitro, NVs significantly reduced the secretion of bone marrow macrophages and proinflammatory cytokines (IL-10) activated by OMVs in primary mice. This finding is consistent with previous findings. Interestingly, IL-10 levels were dose-dependently upregulated in OMV-stimulated macrophages after NV treatment, whereas OMV alone did not alter IL-10 production. It is evident that NVs hold promise for ameliorating septic inflammation and reducing liver injury.
Free DNA (cfDNA) plays a key role in the Toll-like receptor 9 (TLR-9)-mediated proinflammatory pathway, and reducing or eliminating cfDNA can modulate the immune response, thereby reducing organ damage and suppressing cytokine storms during sepsis. To validate this hypothesis, Dawulieti et al108 synthesized three polyethyleneamine (PEI)-functionalized and biodegradable mesoporous silica nanoparticles with different charge densities (MSN-PEI25K, MSN-PEI800 and MSN-NH2) and compared their cfDNA removal effects. The results showed that MSN-PEI 25K had the best anti-inflammatory effect due to its high charge density and strong nucleic acid binding ability. In addition, the toxicity of the three particles was evaluated, and the median inhibitory concentration of macrophages (IC50) of MSN-PEI 25K was 22.98 μg/mL, which was much lower than that of the other two, and all mice in the MSN-PEI 25K and MSN-PEI 800 groups had a 50% mortality rate at a dose of 640 mg/kg, while mice in the PEI group exhibited mortality at a dose of 40 mg/kg. Finally, serum biochemical parameters were analyzed and showed that MSN-PEI 25K significantly reduced serum alanine aminotransferase, aspartate aminotransferase, bilirubin, blood urea nitrogen, creatinine and creatine kinase levels, indicating the protection of liver function and low toxicity of macrophages. This finding suggests that the great research value of these particles as cfDNA scavengers for organ protection and reversing inflammation during sepsis.
Inflammation and oxidative stress trigger sepsis, and suppressing immune hyperactivation has become an important strategy.108,109 It has been found that the incidence of sepsis in animals with tumors (eg melanoma) is much lower than that in normal animals, suggesting that tumors may be able to reduce sepsis-related immune hyperactivation and achieve sepsis relief.110–112 Li et al113 assessed the potential for treating sepsis-associated inflammation and oxidative stress by using hollow fiber tubes to encapsulate tumor cells and loading miRNA into hyaluronic acid-polyethyleneimine nanoparticles to generate exosomes (iExos). In vivo, a “tumor-bearer-sepsis” animal model was developed, and the induced exosomes that were secreted by tumor cells after LPS treatment (iExos) were more effective than normal exosomes (nExo) in ameliorating sepsis. The average particle size of iExos was approximately 137 nm, as determined by DLS and mass spectrometry, and these factors exhibited a disc-like structure and large internal space, which could affect immune and endothelial functions. In vivo, iExo-treated rats had a 50% lower mortality rate and less inflammatory exudate in the abdominal cavity than rats in the model group. Hematoxylin and eosin (H&E)-stained sections showed that the liver, kidney and lung tissue of septic rats in this group were less damaged than those in the other groups. ELISA showed that iExos reduced IL-6 and TNF-α levels in mouse serum. HUVECs and RAW264.7 cells were used to establish an in vitro sepsis-associated inflammation model to assess the effect on macrophages and endothelial cells. The Western blot and ELISA results showed that iExos were not affected by proteinase K treatment and that miRNAs were key substances associated with the amelioration of sepsis-associated inflammation by iExos. The most regulated pathway in macrophages was the TNF pathway, which acted mainly through 7 key miRNAs. Finally, the use of exosomes with specific miRNA ratios was shown to slow the development of sepsis-associated inflammation, suggesting a potential approach to treat sepsis inflammatory storms and protect against organ damage.
There has been a high level of enthusiasm for biomimetic nanomedicines for the treatment of sepsis-associated intrinsic inflammatory diseases in recent years. Molinaro et al114 developed a bionanoparticle (i.e. leukosomes) derived from macrophages, enabling an innovative shift from inert to biological carriers. The researchers extracted membrane proteins, choline-containing phospholipids, and cholesterol from a mouse macrophage cell line (J774 cells) and combined them to form spherical liposome vesicles. The characterization of these vesicles suggested that the average diameter of was 94 nm, and the particles were spherical with a negative charge. Mice were injected intraperitoneally with LPS (15 mg/kg) to induce an inflammatory response and were then injected with leukosomes. Thirty minutes later, in vivo confocal microscopy showed significant accumulation of nanoparticles in the lungs, liver and spleen, with increased accumulation 6 hours after injection, and 66% of mice in the leukosome group survived for 120 hours, indicating the intrinsic targeting properties of leukosomes to inflamed tissues. Later, liver and lung tissues were collected from the mice and analyzed using H&E staining, and the results showed that the organs were lighter in the leukocyte group than in the LPS group, indicating their ability to reduce liver and lung damage. Finally, the enzyme-linked immunosorbent assay (ELISA) and Western blot results showed that white microsomes could attenuate glomerular and tubular injury, reduce liver necrosis, and effectively inhibit the increase in proinflammatory genes (IL-10 and TGF-β). For cellular experiments, peripheral blood was collected from the mice 1 hour after leukocyte injection, and flow cytometric analysis showed that 9% of leukocytes interacted with macrophages and that 24-hour in vitro PS activation of cells with LPS induced significant increases in the proinflammatory genes IL-6 and TNF-α. Consistent with previous findings, the direct role of leukocytes in regulating the expression of pro- and anti-inflammatory cytokines in macrophages was demonstrated. These experimental results provide new ideas for the treatment of liver injury during sepsis.
Conclusion and Future Prospects
Sepsis is a systemic inflammatory response syndrome caused by infection with a high incidence. It has become the main cause of death in intensive care unit (ICU) patients in China and seriously threatens human health. At this stage, there is no specific clinical treatment. Infection and invading germs trigger an inflammatory response in the body and lead to a series of pathophysiological changes during the continuous development of inflammation, including severe sepsis, septic shock, and multiorgan dysfunction syndrome. The liver is one of the most easily damaged target organs in sepsis and is easily overlooked by clinicians. However, the pathogenesis of SRLI is intricate and complex, and various factors interact with each other; therefore, the treatment of sepsis-related liver injury mainly involves controlling the liver inflammatory response and oxidative stress, as well as treating liver microcirculatory coagulation disorders and bacterial translocation. However, many small molecule drugs are underutilized due to their short half-lives and side effects. The continuous development of drug delivery nanosystems has provided new methods for the delivery of small molecule drugs. This paper reviewed the drug delivery nanosystems that had been created to improve liver targeting of SRLI and their antioxidant and anti-inflammatory abilities, and reduce side effects, and increase bioavailability, which are good implications for the treatment of sepsis.
Unfortunately, these promising new discoveries will not be available for clinical application soon. There are several reasons for this. First, drug delivery nanosystems need to successfully cross many barriers before they can reach their targets. For example, during sepsis, neutrophils release extracellular traps (NETs) for a short period of time to capture and kill pathogens, and NPs are easily perceived as “foreign bodies” to be captured and phagocytosed, severely affecting the release of NP-loaded drugs and ultimately leading to therapeutic failure. Several studies have shown that NPs can trigger the formation of NETs and that their surface chemistry has a significant effect on the number of NPs trapped; NETs gradually form and trap more NPs within 1 hour when NPs of different sizes and shapes interact with neutrophils.115–117 Therefore, how to help NPs overcome the immune system and reach the target site smoothly is a question worthy of further thought. Interestingly, dextran- and albumin-coated SPIONs were recently shown to prevent NET formation and vascular occlusion in vivo, suggesting that stabilizing NPs with a biocompatible layer may lead to inert behavior and phagocytosis-induced clearance118,119,It is clear that NPs can be important in designing drug delivery nanosystems by switching the surface chemistry, size and shape. Second, due to multiple organ damage, the existing nanosystems are slightly singular in function and do not address the multiple levels of damage in sepsis. The time frame for toxicity and targeting studies on the liver is relatively short, and continuous structural optimization and long-term studies are needed to better understand the effects of nanoparticles. Fortunately, lipids are biodegradable, and their use in nanomedicines offers more possibilities for drug encapsulation and reducing toxicity. Finally, the translational gap between clinical studies and clinical trials, such as ex vivo and animal studies, is another challenge. A characteristic factor was the “ACCESS” trial.120 This trial compared Eritoran (a TLR4 antagonist) with placebo in a double-blind randomized controlled trial. The results showed that patients in the TLR4 antagonist-treated group did not have a lower mortality rate at 28 days than those in the placebo group. In addition to the fact that the trial did not include any NP-based systems, it highlights the difficulty of using laboratory results for clinical practice. Whether nanoparticles can be controlled for mass production remains to be determined.
There is no doubt that this is a golden time to explore the field of nanomedicine to treat, diagnose and manage SRLI. Drug delivery nanosystems are emerging as innovative strategies to provide more efficient and safer routes for crossing biological barriers and protecting organ function. As nanotechnology continues to develop and research continues, nanodrug delivery systems have a very bright future in the treatment of liver injury associated with sepsis.
Abbreviations
SRLI, Sepsis related liver injury; CLP, Cecal ligation and puncture; KC, Kupffer cell, S. aureus, Staphylococcus aureus; HSC, Hepatic stellate cell; E. coli, Escherichia coli; MS, Microspheres; TLN, Triple-layered nanogel; AMP, Antimicrobial peptide; MSCs, Bone mesenchymal stem cells; ICAM-1, Intercellular cell adhesion molecule-1; EVs, Extracellular vesicles; ROS, Reactive oxygen species; NVs, Nanovesicles; RNS, Reactive Nitrogen Species; OMVs, Outer membrane vesicles; PMN, Polymorphonuclear neutrophil; TNF-α, Tumor necrosis factor-α; DOX, Doxorubicin; IL-6, Interleukin-6; hyd, hydrozone linker; NPs, Nanoparticles; BSA, Bovine Serum Albumin; cfDNA, Cell free DNA; SPIONs, Superparamagnetic iron oxide nanoparticles; PEI, Polyethylenimine; IL-10, Interleukin-10; iExo, Induced exosomes; Cav1, Caveolin-1; miRNA, MicroRNA; ALT, Alanine transaminase; ELISA, Enzyme-linked immunosorbent assay; AST, Aspartate transaminase; NETs, Neutrophilic granulocytes; Mel, Melatonin; MSN, Mesoporous silicon; MDA, Malondialdehyde; RNPN, Redox nanoparticle; HUVECs, Human Umbilical Vein Endothelial Cells; BM, Bovine serum albumin reduced manganese dioxide; SOD, Superoxide Dismutase; CuO, Copper oxide; Cap, Calcium phosphate; TiO2, Titanium dioxide; AERL, Rubescens leaves; Ag, Argentum; CeO, Cerium oxide; WS2, Full tungsten disulfide; CeO2, Full Cerium(IV) oxide; MoSe2, Molybdenum diselenide; WSe2, Tungsten diselenide; Fcc, Face-centered cubic; PAF, Platelet-Activating Factor; MIC, Minimum inhibitory concentration; TLR, Toll-like receptors; TPL, Tempol; ER-β, Estrogen Receptor β; Caspase-1, Cysteinyl aspartate specific proteinase-1; HL-60, a human promyelocytic leukemia cell line; NK, natural killer cell.
Acknowledgments
This study was supported by following:
Cultivating program of Clinical Science and technology innovation for Dragon Medical Scholars, Longhua Hospital, Shanghai University of Traditional Chinese Medicine (Grant NO.PY20220112011), The fifth batch of “Longyi Scholar” Clinical Scientific and technological Innovation training Project of Longhua Hospital affiliated to Shanghai University of Traditional Chinese Medicine(PY2022011) and Excellent Reserve talents of traditional Chinese Medicine in Shanghai University of traditional Chinese Medicine(2020).The university level scientific research project of Zhejiang Shuren University (2022R005), Training plan for leading talents from Universities in Zhejiang Province.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Mushtaq A, Kazi F. Updates in sepsis management. Lancet Infect Dis. 2022;22(1):24. doi:10.1016/S1473-3099(21)00773-8
2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200–211. doi:10.1016/S0140-6736(19)32989-7
3. Guo H, Ni M, Xu J, et al. Transcriptional enhancement of GBP-5 by BATF aggravates sepsis-associated liver injury via NLRP3 inflammasome activation. FASEB J. 2021;35(6):e21672. doi:10.1096/fj.202100234R
4. Kaur S, Hussain S, Kolhe K, et al. Elevated plasma ICAM1 levels predict 28-day mortality in cirrhotic patients with COVID-19 or bacterial sepsis. JHEP Reports. 2021;3(4):100303. doi:10.1016/j.jhepr.2021.100303
5. Cao E, Luan ZG, Wang L, Liu YN, Hu B, Ma XC. Clinical characteristics and prognosis analysis of sepsis-related liver injury. Chin Pract Inter Med Miscellan. 2019;39(02):163–167.
6. Strnad P, Tacke F, Koch A, Trautwein C. Liver — guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol. 2017;14(1):55–66. doi:10.1038/nrgastro.2016.168
7. Van der Merwe S, Chokshi S, Bernsmeier C, Albillos A. The multifactorial mechanisms of bacterial infection in decompensated cirrhosis. J Hepatol. 2021;75(Suppl 1):S82–S100. doi:10.1016/j.jhep.2020.11.029
8. Song H, Zhang X, Zhai R, et al. Metformin attenuated sepsis-associated liver injury and inflammatory response in aged mice. Bioengineered. 2022;13(2):4598–4609. doi:10.1080/21655979.2022.2036305
9. Zhibao S, Qinghua A, Yuming Z, Yue C. The functional changes of heart and microcirculation in septic shock patients and the Hemodynamics effect of β-blockers. Chin J Hospital Infect. 2016;26(07):1528–1530.
10. Beurskens Danielle MH, Huckriede JP, Schrijver R, Hemker HC, Reutelingsperger CP, Nicolaes Gerry AF. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120(10):1371–1383. doi:10.1055/s-0040-1715460
11. Ansell J, Laulicht BE, Bakhru SH, et al. Ciraparantag, an anticoagulant reversal drug: mechanism of action, pharmacokinetics, and reversal of anticoagulants. Blood. 2021;137(1):115–125. doi:10.1182/blood.2020007116
12. Shi Y, Zhu M-L, Wu Q, Huang Y, Xu X-L, Chen W. The potential of drug delivery nanosystems for sepsis treatment. J Inflamm Res. 2021;14:7065–7077. doi:10.2147/JIR.S339113
13. Rhee C, Kadri SS, Dekker JP, et al.; CDC Prevention Epicenters Program. Prevalence of antibiotic-resistant pathogens in culture-proven sepsis and outcomes associated with inadequate and broad-spectrum empiric antibiotic use. JAMA Netw Open. 2020;3(4):e202899. doi:10.1001/jamanetworkopen.2020.2899
14. Chauhan N, Tiwari S, Jain U. Potential biomarkers for effective screening of neonatal sepsis infections: an overview. Microb Pathog. 2017;107:234–242. doi:10.1016/j.micpath.2017.03.042
15. Ji Y, Yin Y, Li Z, Zhang W. Gut microbiota-derived components and metabolites in the progression of Non-Alcoholic Fatty Liver Disease (NAFLD). Nutrients. 2019;11(8):1712. doi:10.3390/nu11081712
16. Zhang X, Liu H, Hashimoto K, Yuan S, Zhang J. The gut–liver axis in sepsis: interaction mechanisms and therapeutic potential. Crit Care. 2022;26(1):213. doi:10.1186/s13054-022-04090-1
17. Patil YP, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8–18. doi:10.1016/j.chemphyslip.2013.10.011
18. De Jong DE. Drug delivery and nanoparticles: applications and hazards. Int J Nanomedicine. 2008;3(2):133–149. doi:10.2147/IJN.S596
19. Rajpoot K. Solid lipid nanoparticles: a promising nanomaterial in drug delivery. Curr Pharm Des. 2019;25(37):3943–3959.
20. Lin M, Dai Y, Xia F, Zhang X. Advances in non-covalent crosslinked polymer micelles for biomedical applications. Mater Sci Eng C Mater Biol Appl. 2021;119:111626.
21. Jin X, Sun P, Tong G, Zhu X. Star polymer-based unimolecular micelles and their application in bio-imaging and diagnosis. Biomaterials. 2018;178:738–750.
22. Wang S, Gao J, Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(2):e1523.
23. Surman M, Drożdż A, Stępień E, Przybyło M. Extracellular vesicles as drug delivery systems – methods of production and potential therapeutic applications. Curr Pharm Des. 2019;25(2):132–154.
24. Jin F, Liu D, Yu H, et al. Sialic Acid-Functionalized PEG-PLGA microspheres loading mitochondrial-targeting-modified curcumin for acute lung injury therapy. Mol Pharm. 2019;16(1):71–85.
25. Kasuya T, Kuroda S. Nanoparticles for human liver-specific drug and gene delivery systems: in vitro and in vivo advances. Expert Opin Drug Deliv. 2009;6(1):39–52.
26. Shao D, Lu MM, Zhao YW, et al. The shape effect of magnetic mesoporous silica nanoparticles on endocytosis, biocompatibility and biodistribution. Acta Biomater. 2017;49:531–540.
27. Fan X, Fan J, Wang X, Wu P, Wu G. S-thanatin functionalized liposome potentially targeting on Klebsiella pneumoniae and its application in sepsis mouse model. Front Pharmacol. 2015;27(6):249.
28. Pant A, Mackraj I, Govender T. Advances in sepsis diagnosis and management: a paradigm shift towards nanotechnology. J Biomed Sci. 2021;28(1):6.
29. Jiang S, Li S, Hu J, et al. Combined delivery of angiopoietin-1 gene and simvastatin mediated by anti-intercellular adhesion molecule-1 antibody-conjugated ternary nanoparticles for acute lung injury therapy. Nanomedicine. 2019;15(1):25–36.
30. Qi J, Li W, Xu X, et al. Cyto-friendly polymerization at cell surfaces modulates cell fate by clustering cell-surface receptors. Chem Sci. 2020;11(16):4221–4225.
31. Silva CMS, Wanderley CWS, Veras FP, et al. Gasdermin D inhibition prevents multiple organ dysfunction during sepsis by blocking NET formation. Blood. 2021;138(25):2702–2713.
32. Meghraoui-Kheddar A, Chousterman BG, Guillou N, et al. Two new neutrophil subsets define a discriminating sepsis signature. Am J Respir Crit Care Med. 2022;205(1):46–59.
33. Margraf A, Lowell CA, Zarbock A. Neutrophils in acute inflammation: current concepts and translational implications. Blood. 2022;139(14):2130–2144.
34. Zhang CY, Dong X, Gao J, Lin W, Liu Z, Wang Z. Nanoparticle-induced neutrophil apoptosis increases survival in sepsis and alleviates neurological damage in stroke. Sci Adv. 2019;5(11):eaax7964.
35. Deretic V. Autophagy in inflammation, infection, and immunometabolism. Immunity. 2021;54(3):437–453.
36. Qian H, Chao X, Williams J, et al. Autophagy in liver diseases: a review. Mol Aspects Med. 2021;82:100973.
37. Ueno T, Komatsu M. Autophagy in the liver: functions in health and disease. Nat Rev Gastroenterol Hepatol. 2017;14(3):170–184.
38. Yang Z, Zhang J, Wang Y, Lu J, Sun Q. Caveolin-1 deficiency protects mice against carbon tetrachloride-induced acute liver injury through regulating polarization of hepatic macrophages. Front Immunol. 2021;12:713808.
39. Li G, Wang B, Ding X, Zhang X, Tang J, Lin H. Plasma extracellular vesicle delivery of miR-210-3p by targeting ATG7 to promote sepsis-induced acute lung injury by regulating autophagy and activating inflammation. Exp Mol Med. 2021;53(7):1180–1191.
40. Hwang YH, Kim YJ, Lee DY. Hepatic and renal cellular cytotoxic effects of heparin-coated superparamagnetic Iron oxide nanoparticles. Biomater Res. 2021;25(1):36.
41. Grippin AJ, Wummer B, Wildes T, et al. Dendritic cell-activating magnetic nanoparticles enable early prediction of antitumor response with magnetic resonance imaging. ACS Nano. 2019;13(12):13884–13898.
42. Mou Y, Hou Y, Chen B, et al. In vivo migration of dendritic cells labeled with synthetic superparamagnetic iron oxide. Int J Nanomedicine. 2011;6:2633–2640.
43. Xu Y, Li Y, Liu X, et al. SPIONs enhances IL-10-producing macrophages to relieve sepsis via Cav1-Notch1/HES1-mediated autophagy. Int J Nanomedicine. 2019;14:6779–6797.
44. Koide H, Okishima A, Hoshino Y, et al. Synthetic hydrogel nanoparticles for sepsis therapy. Nat Commun. 2021;12(1):5552.
45. Wong F, Piano S, Singh V, et al.; International Club of Ascites Global Study Group. Clinical features and evolution of bacterial infection-related acute-on-chronic liver failure. J Hepatol. 2021;74(2):330–339.
46. Mao D, Hu F, Kenry JS, et al. Metal-organic-framework-assisted in vivo bacterial metabolic labeling and precise antibacterial therapy. Adv Mater. 2018;30(18):e1706831.
47. Jiang J, Xu Z, Chen J, et al. Staphylococcus aureus-targeting peptide/surfactant assemblies for antibacterial therapy. Colloids Surf B Biointerfaces. 2022;214:112444.
48. Bajaj JS, Kamath PS, Reddy KR. The evolving challenge of infections in cirrhosis. N Engl J Med. 2021;384(24):2317–2330.
49. Wu B, Lin L, Zhou F, Wang X. Precise engineering of neutrophil membrane coated with polymeric nanoparticles concurrently absorbing of proinflammatory cytokines and endotoxins for management of sepsis. Bioprocess Biosyst Eng. 2020;43(11):2065–2074.
50. Dong X, Gao J, Zhang CY, Hayworth C, Frank M, Wang Z. Neutrophil membrane-derived nanovesicles alleviate inflammation to protect mouse brain injury from ischemic stroke. ACS Nano. 2019;13(2):1272–1283.
51. Yang Y, Ding Y, Fan B, et al. Inflammation-targeting polymeric nanoparticles deliver sparfloxacin and tacrolimus for combating acute lung sepsis. J Control Release. 2020;10(321):463–474.
52. Wang X, Mohammad IS, Fan L, et al. Delivery strategies of amphotericin B for invasive fungal infections. Acta Pharm Sin B. 2021;11(8):2585–2604.
53. Xiong MH, Bao Y, Yang XZ, Wang YC, Sun B, Wang J. Lipase-sensitive polymeric triple-layered nanogel for “on-demand” drug delivery. J Am Chem Soc. 2012;134(9):4355–4362.
54. Mohammadi A, Sharifi A, Pourpaknia R, Mohammadian S, Sahebkar A. Manipulating macrophage polarization and function using classical HDAC inhibitors: implications for autoimmunity and inflammation. Crit Rev Oncol Hematol. 2018;128:1–18.
55. Zhang CY, Gao J, Wang Z. Bioresponsive nanoparticles targeted to infectious microenvironments for sepsis management. Adv Mater. 2018;30(43):e1803618.
56. Sims KR, Liu Y, Hwang G, Jung HI, Koo H, Benoit DSW. Enhanced design and formulation of nanoparticles for anti-biofilm drug delivery. Nanoscale. 2018;11(1):219–236.
57. Xiong M, Bao Y, Xu X, et al. Selective killing of Helicobacter pylori with pH-responsive helix-coil conformation transitionable antimicrobial polypeptides. Proc Natl Acad Sci U S A. 2017;114(48):12675–12680.
58. Mohanty A, Fatrekar AP, Vernekar AA. All Ag nanoparticles are not the same: covalent interactions between Ag nanoparticles and nitrile groups help combat drug- and Ag-resistant bacteria. ChemMedChem. 2021;16(23):3545–3547.
59. Casals G, Perramón M, Casals E, et al. Cerium oxide nanoparticles: a new therapeutic tool in liver diseases. Antioxidants. 2021;10(5):660.
60. Zhang W. The mitophagy receptor FUN14 domain-containing 1 (FUNDC1): a promising biomarker and potential therapeutic target of human diseases. Genes Dis. 2020;8(5):640–654.
61. Huff LA, Yan S, Clemens MG. Mechanisms of Ataxia Telangiectasia Mutated (ATM) control in the DNA damage response to oxidative stress, epigenetic regulation, and persistent innate immune suppression following sepsis. Antioxidants. 2021;10(7):1146.
62. Yang J, Liu H, Han S, et al. Melatonin pretreatment alleviates renal ischemia-reperfusion injury by promoting autophagic flux via TLR4/MyD88/MEK/ERK/mTORC1 signaling. FASEB J. 2020;34(9):12324–12337.
63. Sutton SS, Magagnoli J, Cummings TH, Hardin JW. Melatonin use and the risk of 30-day mortality among US veterans with sepsis: a retrospective study. J Pineal Res. 2022;73(2):e12811.
64. Hasan ZT, Atrakji DM, Mehuaiden DAK. The effect of melatonin on thrombosis, sepsis and mortality rate in COVID-19 patients. Int J Infect Dis. 2022;114:79–84.
65. Jin Y, Wang H, Yi K, et al. Applications of nanobiomaterials in the therapy and imaging of acute liver failure. Nanomicro Lett. 2020;13(1):25.
66. Chen G, Deng H, Song X, et al. Reactive oxygen species-responsive polymeric nanoparticles for alleviating sepsis-induced acute liver injury in mice. Biomaterials. 2017;144:30–41.
67. Leong XF. Lipid oxidation products on inflammation-mediated hypertension and atherosclerosis: a mini review. Front Nutr. 2021;30(8):717740.
68. Hsu CG, Chávez CL, Zhang C, Sowden M, Yan C, Berk BC. The lipid peroxidation product 4-hydroxynonenal inhibits NLRP3 inflammasome activation and macrophage pyroptosis. Cell Death Differ. 2022;29(9):1790–1803.
69. Sun X, Seidman JS, Zhao P, et al. Neutralization of oxidized phospholipids ameliorates non-alcoholic steatohepatitis. Cell Metab. 2020;31(1):189–206.e8.
70. Guan Y, Yao W, Yi K, et al. Nanotheranostics for the management of hepatic ischemia-reperfusion injury. Small. 2021;17(23):e2007727.
71. Yu H, Jin F, Liu D, et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics. 2020;10(5):2342–2357.
72. Kadono K, Kageyama S, Nakamura K, et al. Myeloid Ikaros-SIRT1 signaling axis regulates hepatic inflammation and pyroptosis in ischemia-stressed mouse and human liver. J Hepatol. 2022;76(4):896–909.
73. Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14(7):417–427.
74. Çakır M, Tekin S, Okan A, Çakan P, Doğanyiğit Z. The ameliorating effect of cannabinoid type 2 receptor activation on brain, lung, liver and heart damage in cecal ligation and puncture-induced sepsis model in rats. Int Immunopharmacol. 2020;78:105978.
75. Ye M, Zhao Y, Wang Y, et al. NAD(H)-loaded nanoparticles for efficient sepsis therapy via modulating immune and vascular homeostasis. Nat Nanotechnol. 2022;17(8):880–890.
76. Manne ND, Arvapalli R, Nepal N, et al. Therapeutic potential of cerium oxide nanoparticles for the treatment of peritonitis induced by polymicrobial insult in Sprague-Dawley rats. Crit Care Med. 2015;43(11):e477–e489.
77. Soh M, Kang DW, Jeong HG, et al. Ceria-zirconia nanoparticles as an enhanced multi-antioxidant for sepsis treatment. Angew Chem Int Ed Engl. 2017;56(38):11399–11403.
78. Selvaraj V, Nepal N, Rogers S, et al. Inhibition of MAP kinase/NF-kB mediated signaling and attenuation of lipopolysaccharide induced severe sepsis by cerium oxide nanoparticles. Biomaterials. 2015;59:160–171.
79. Fehaid A, Taniguchi A. Size-dependent effect of silver nanoparticles on the tumor necrosis factor α-induced DNA damage response. Int J Mol Sci. 2019;20(5):1038.
80. Yim D, Lee DE, So Y, et al. Sustainable nanosheet antioxidants for sepsis therapy via scavenging intracellular reactive oxygen and nitrogen species. ACS Nano. 2020;14(8):10324–10336.
81. Peng Z, Zhang M, Ouyang M, et al. Protective effects of myocardium-targeted nanoparticles loaded L-arginineon on sepsis-induced myocardial injury. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2020;32(8):953–959. Chinese. doi:10.3760/cma.j.cn121430-20200217-00168
82. Feliciano CP, Tsuboi K, Suzuki K, Kimura H, Nagasaki Y. Long-term bioavailability of redox nanoparticles effectively reduces organ dysfunctions and death in whole-body irradiated mice. Biomaterials. 2017;129:68–82.
83. Rajendrakumar SK, Revuri V, Samidurai M, et al. Peroxidase-mimicking nanoassembly mitigates lipopolysaccharide-induced endotoxemia and cognitive damage in the brain by impeding inflammatory signaling in macrophages. Nano Lett. 2018;18(10):6417–6426.
84. Lee DY, Kang S, Lee Y, et al. PEGylated bilirubin-coated iron oxide nanoparticles as a biosensor for magnetic relaxation switching-based ROS detection in whole blood. Theranostics. 2020;10(5):1997–2007.
85. Liu H, Lai W, Liu X, et al. Exposure to copper oxide nanoparticles triggers oxidative stress and endoplasmic reticulum (ER)-stress induced toxicology and apoptosis in male rat liver and BRL-3A cell. J Hazard Mater. 2021;401:123349.
86. Abbasi-Oshaghi E, Mirzaei F, Pourjafar M. NLRP3 inflammasome, oxidative stress, and apoptosis induced in the intestine and liver of rats treated with titanium dioxide nanoparticles: in vivo and in vitro study. Int J Nanomedicine. 2019;14:1919–1936.
87. Zhou H, Fan Z, Li PY, et al. Dense and dynamic polyethylene glycol shells cloak nanoparticles from uptake by liver endothelial cells for long blood circulation. ACS Nano. 2018;12(10):10130–10141.
88. Fang S, Li P, Zhu C, Han X, Bao P, Guo W. Research progress of ulinastatin in the treatment of liver diseases. Int J Clin Exp Pathol. 2020;13(11):2720–2726.
89. Wang Y, Tang J, Zhu H, et al. Aqueous extract of Rabdosia rubescens leaves: forming nanoparticles, targeting P-selectin, and inhibiting thrombosis. Int J Nanomedicine. 2015;4(10):6905–6918.
90. Li Y, Wan D, Luo X, et al. Circulating histones in sepsis: potential outcome predictors and therapeutic targets. Front Immunol. 2021;24(12):650184.
91. Zetoune FS, Ward PA. Role of complement and histones in sepsis. Front Med. 2020;23(7):616957.
92. Frydman GH, Tessier SN, Wong KHK, et al. Megakaryocytes contain extranuclear histones and may be a source of platelet-associated histones during sepsis. Sci Rep. 2020;10(1):4621.
93. Pan B, Li Y, Liu Y, Wang W, Huang G, Ouyang Y. Circulating CitH3 Is a reliable diagnostic and prognostic biomarker of septic patients in acute pancreatitis. Front Immunol. 2021;12:766391.
94. Tian Y, Russo RM, Li Y, et al. Serum citrullinated histone H3 concentrations differentiate patients with septic verses non-septic shock and correlate with disease severity. Infection. 2021;49(1):83–93.
95. Xie C, Halegoua-DeMarzio D. Role of probiotics in non-alcoholic fatty liver disease: does gut microbiota matter? Nutrients. 2019;11(11):2837.
96. Sun J, Zhang J, Wang X, et al. Gut-liver crosstalk in sepsis-induced liver injury. Crit Care. 2020;24(1):614.
97. Adiliaghdam F, Cavallaro P, Mohad V, et al. Targeting the gut to prevent sepsis from a cutaneous burn. JCI Insight. 2020;5(19):e137128.
98. Acharya D, Singha KM, Pandey P, Mohanta B, Rajkumari J, Singha LP. Shape dependent physical mutilation and lethal effects of silver nanoparticles on bacteria. Sci Rep. 2018;8(1):201.
99. Agnihotri S, Mukherji S, Mukherji S. Size-controlled silver nanoparticles synthesized over the range 5–100 nm using the same protocol and their antibacterial efficacy. RSC Adv. 2014;4:3974–3983.
100. Lertwattanachai T, Montakantikul P, Tangsujaritvijit V, et al. Clinical outcomes of empirical high-dose meropenem in critically ill patients with sepsis and septic shock: a randomized controlled trial. J Intensive Care. 2020;15(8):26.
101. Kothekar AT, Divatia JV, Myatra SN, et al. Clinical pharmacokinetics of 3-h extended infusion of meropenem in adult patients with severe sepsis and septic shock: implications for empirical therapy against Gram-negative bacteria. Ann Intensive Care. 2020;10(1):4.
102. Memar MY, Yekani M, Ghanbari H, Shahi S, Sharifi S, Maleki Dizaj S. Biocompatibility, cytotoxicity and antibacterial effects of meropenem-loaded mesoporous silica nanoparticles against carbapenem-resistant Enterobacteriaceae. Artif Cells Nanomed Biotechnol. 2020;48(1):1354–1361.
103. Fan W, Zhang S, Wu Y, et al. Genistein-derived ROS-responsive nanoparticles relieve colitis by regulating mucosal homeostasis. ACS Appl Mater Interfaces. 2021;13(34):40249–40266.
104. Hou X, Zhang X, Zhao W, et al. Vitamin lipid nanoparticles enable adoptive macrophage transfer for the treatment of multidrug-resistant bacterial sepsis. Nat Nanotechnol. 2020;15(1):41–46.
105. Yin H, Zhou M, Chen X, et al. Fructose-coated Ångstrom silver prevents sepsis by killing bacteria and attenuating bacterial toxin-induced injuries. Theranostics. 2021;11(17):8152–8171.
106. You J, Fu Z, Zou L. Mechanism and potential of extracellular vesicles derived from mesenchymal stem cells for the treatment of infectious diseases. Front Microbiol. 2021;26(12):761338.
107. Park KS, Svennerholm K, Shelke GV, et al. Mesenchymal stromal cell-derived nanovesicles ameliorate bacterial outer membrane vesicle-induced sepsis via IL-10. Stem Cell Res Ther. 2019;10(1):231.
108. Dawulieti J, Sun M, Zhao Y, et al. Treatment of severe sepsis with nanoparticulate cell-free DNA scavengers. Sci Adv. 2020;6(22):eaay7148.
109. Copaescu A, Smibert O, Gibson A, Phillips EJ, Trubiano JA. The role of IL-6 and other mediators in the cytokine storm associated with SARS-CoV-2 infection. J Allergy Clin Immunol. 2020;146(3):518–534.e1.
110. Van de Louw A, Cohrs A, Leslie D. Incidence of sepsis and associated mortality within the first year after cancer diagnosis in middle aged adults: a US population based study. PLoS One. 2020;15(12):e0243449.
111. Te Marvelde L, Whitfield A, Shepheard J, Read C, Milne RL, Whitfield K. Authors’ response to Sepsis in cancer: a question of definition. Aust N Z J Public Health. 2020;44(3):246.
112. Daassi D, Mahoney KM, Freeman GJ. The importance of exosomal PDL1 in tumour immune evasion. Nat Rev Immunol. 2020;20(4):209–215.
113. Li Y, Zhang H, Chen C, et al. Biomimetic immunosuppressive exosomes that inhibit cytokine storms contribute to the alleviation of sepsis. Adv Mater. 2022;34(19):e2108476.
114. Molinaro R, Pastò A, Corbo C, et al. Macrophage-derived nanovesicles exert intrinsic anti-inflammatory properties and prolong survival in sepsis through a direct interaction with macrophages. Nanoscale. 2019;11(28):13576–13586.
115. Plana E, Oto J, Medina P, Fernández-Pardo Á, Miralles M. Novel contributions of neutrophils in the pathogenesis of abdominal aortic aneurysm, the role of neutrophil extracellular traps: a systematic review. Thromb Res. 2020;194:200–208.
116. Grégoire M, Uhel F, Lesouhaitier M, et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur Respir J. 2018;52(2):1702590.
117. Wang K, Lei Y, Xia D, et al. Neutrophil membranes coated, antibiotic agent loaded nanoparticles targeting to the lung inflammation. Colloids Surf B Biointerfaces. 2020;188:110755.
118. Bilyy R, Unterweger H, Weigel B, et al. Inert coats of magnetic nanoparticles prevent formation of occlusive intravascular co-aggregates with neutrophil extracellular traps. Front Immunol. 2018;2(9):2266.
119. Papafilippou L, Claxton A, Dark P, Kostarelos K, Hadjidemetriou M. Nanotools for sepsis diagnosis and treatment. Adv Healthc Mater. 2021;10(1):e2001378.
120. Opal SM, Laterre PF, Francois B, et al.; ACCESS Study Group. Effect of eritoran, an antagonist of MD2-TLR4, on mortality in patients with severe sepsis: the ACCESS randomized trial. JAMA. 2013;309(11):1154–1162.



