Algae-Based Microbots Deliver Cancer Drugs Directly to Lung Tumors
Want to listen to this article for FREE?
Complete the form below to unlock access to ALL audio articles.
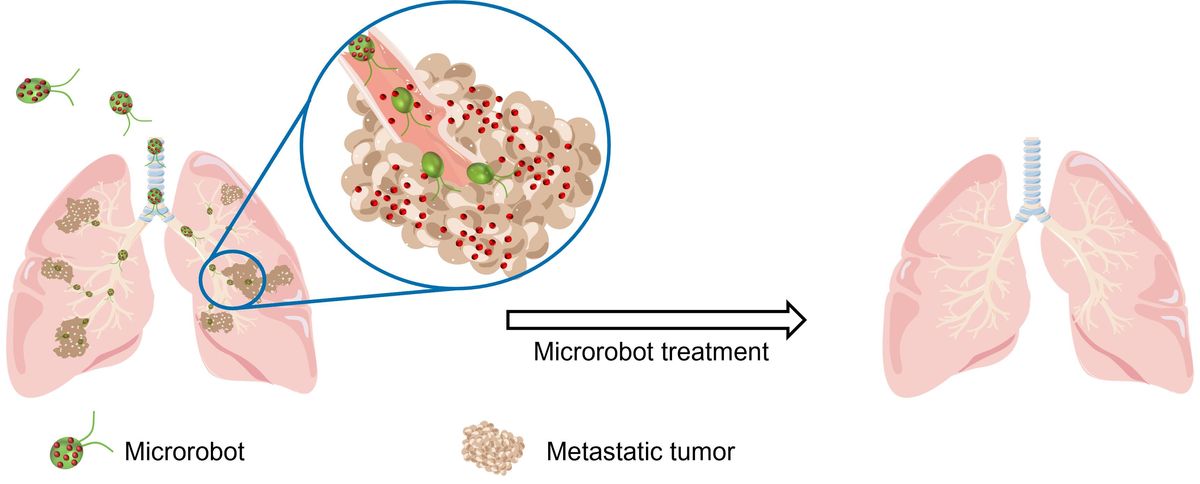
Combining biology and nanotechnology, researchers have developed algae-based “microbots” to deliver drugs directly to lung tumors in mice – with the approach showing promise for reducing tumor growth and increasing survival.
Improving drug delivery to lung metastases
Cancers that spread – or metastasize – from other parts of the body into the lungs can represent challenging targets for treatment. Commonly used chemotherapy drugs can fail to directly reach the lungs, requiring new approaches to deliver therapies more effectively.
In a new study, researchers from the University of California, San Diego (UCSD) built upon previous research in which they developed a type of microscopic robot, or “microbot”, to help treat pneumonia in mice – adapting it to target tumors in the lungs instead. The study is published in Science Advances.
Want more breaking news?
Subscribe to Technology Networks’ daily newsletter, delivering breaking science news straight to your inbox every day.
Formed from algae cells speckled with antibiotic-filled nanoparticles, the microbots could swim around the mouse lung and deliver antibiotics to the pneumonia-causing bacteria. All the microbot-treated mice survived, whereas untreated mice succumbed to their infection within three days.
“Those were the first microrobots to be safely tested in the lungs of live animals,” said the study’s co-senior author Joseph Wang, SAIC Endowed Chair in the Department of Nanoengineering at UCSD.
Assembling a cancer-fighting “microbot”
The microbots are built from Chlamydomonas reinhardtii, a species of green algae recognized as safe for use by the US Food and Drug Administration (FDA), which provide their movement. The researchers tweaked the microbots’ components and tailored them to fight the spread of cancer cells in the lungs instead.
Attached to their surface are nanoparticles made from tiny biodegradable polymer spheres loaded with doxorubicin, a common chemotherapy drug. These nanoparticles are coated with red blood cell membranes to protect them against the immune system, and to provide a type of “camouflage” that aids their survival in the lungs long enough to deliver their anti-cancer payload.
The researchers tested the microbots in mouse models of melanoma that had spread to the lungs, delivering them via a small tube inserted into the windpipe.
Treated mice had a median survival of 37 days, compared to 27 days for the untreated mice. Treated mice also survived longer than those that received either the drug only or drug-filled nanoparticles without the algae.
Illustration of the microrobots being administered through the trachea to treat metatstatic tumors in the lungs. Credit: Zhengxing Li
“We demonstrate that this is a platform technology that can actively and efficiently deliver therapeutics throughout the entire lung tissue to combat different types of deadly diseases in the lungs,” said co-senior author Liangfeng Zhang, the Joan and Irwin Jacobs Chancellor Professor at UCSD.
“The active swimming motion of the microrobots significantly improved distribution of the drug to the deep lung tissue, while prolonging retention time,” said Zhengxing Li, nanoengineering PhD student and co-first author of the study. “This enhanced distribution and prolonged retention time allowed us to reduce the required drug dosage, potentially reducing side effects while maintaining high survival efficacy.”
Future work for the team includes progressing to studies of the microbot treatment in larger animals, hoping to pave the way for human trials.
Reference: Zhang F, Guo Z, Li Z, et al. Biohybrid microrobots locally and actively deliver drug-loaded nanoparticles to inhibit the progression of lung metastasis. Sci Adv. 2024;10(24):eadn6157. doi: 10.1126/sciadv.adn6157
This article is a rework of a press release issued by the University of California, San Diego. Material has been edited for length and content.



