MicroRNAs in Hepatocellular Carcinoma: Insights into Regulatory Mechanisms, Clinical Significance, and Therapeutic Potential
Introduction
Primary liver cancer is one of the most common malignant tumors and the third leading cause of cancer-related death globally.1,2 HCC accounts for 75% to 85% of primary liver cancer and has become the fourth most common malignant tumor and the second cause of cancer-related death in China.3 Main treatments of HCC include surgical resection,4 liver transplantation, radiofrequency ablation (RFA), transarterial chemoembolization (TACE),5 transarterial embolization (TAE), radiation therapy, molecular targeted therapy.6 The radiomic immunoscore (RIS), a new radiomic model, not only showed high accuracy in predicting TIME status in the testing cohort (area under the curve = 0.753) but also the capability of predicting therapeutic response to anti-programmed cell death 1 (PD-1) immunotherapy.7 Jiedu Recipe (JR), a Chinese herbal remedy, can prolong overall survival time and decrease recurrence and metastasis rates in patients with HCC via abating the Wnt/β-catenin pathway under hypoxic conditions.8 Great breakthroughs have been made in treating HCC with the emergence of new or multi-modal treatment approaches. However, the prognosis of HCC remains poor, especially in advanced patients, due to the lack of biomarkers for prognosis and treatment response.9 Therefore, deepening the molecular mechanisms of HCC pathogenesis and searching for biomarkers for early diagnosis is of great significance for the prevention and treatment of HCC.
There has been a paradigm shift in treating malignancies with the further study of immuno-oncology.10 Since immune checkpoint inhibitors (ICIs) were approved, immunotherapy is considered a new-generation therapy.11 TIME, angiogenesis, EMT, and drug resistance are the key factors affecting the prognosis of HCC. The TIME includes tumor cells, immune cells, cytokines, etc. The interactions and function of these components determine antitumor immune efficacy.12 Consequently, it is indispensable to elucidate the role of TIME in tumor progression.13 Angiogenesis is a complex process of new and abnormal blood vessel network formation.14 It not only delivers oxygen and nutrients but also transports tumor cells, which plays an important role in tumor growth and metastasis.15 EMT is a complex biological process in which epithelial cells are transformed into cells with mesenchymal phenotypes, which plays a vital role in the malignant features of cancers, such as migration, invasion, metastasis, stem-like properties, and drug resistance.16 Therefore, exploring mechanisms of tumor invasion-metastasis cascade responses is significant for treating tumors.17 Tumor metabolism is characterized by active “aerobic glycolysis”, which can rapidly provide ATP and biosynthetic materials for tumors.18 Even in the presence of sufficient oxygen, tumors prefer ‘aerobic glycolysis’. Studies have shown that metabolic reprogramming of tumors is a survival strategy to adapt to harsh environments with limited glucose and oxygen supply, which is considered a hallmark of tumors and is associated with poor prognosis.19 What is unexpected is that mortality due to drug resistance accounts for more than 90% of cancer-specific mortality.20
MiRNAs, small non-coding RNAs (ncRNAs) about 22 nucleotides in size, play a role in gene expression regulation and almost all major cellular biological processes by targeting the 3′UTR of mRNA. Abnormal expression and dysregulation of miRNAs contributes to tumor development and progression and influences drug resistance in HCC. Accordingly, miRNAs have been extensively investigated as both biomarkers and therapeutic targets as oncogenes or tumor suppressors.21 To date, approximately 2000 human miRNA precursor genes have been annotated in miRBase. Almost 30% of human genes are regulated by miRNAs. Hereafter, we will focus on the latest findings on miRNAs involved in cellular biological processes, including TIME, angiogenesis, EMT, invasion, metastasis, metabolism, and drug resistance, and review the potential value of miRNAs as novel biomarkers and therapeutic candidates for HCC. Then, we will review the main therapeutic strategies of HCC-targeted miRNAs.
Regulatory Mechanisms of MiRNAs in HCC
MiRNAs and TIME
The TIME in HCC involves interactions among tumor cells like HCC cells, the blood arteries around them, stromal cells, immune cells, cytokines, etc. These interactions are essential for tumorigenesis, angiogenesis, and metastasis.22 Evidence suggests that miRNAs play an important role in the TIME of HCC by changing immune phenotypes, hypoxic conditions, and acidification, as well as angiogenesis and extracellular matrix components.23 TIME-based HCC treatment strategies have attracted more and more interest from scholars.24,25 This section comprehensively reviews the roles and molecular mechanisms of miRNAs in regulating immune cell subsets and tumor immune responses in the TIME.
T Cells
CD8+ T Cells
CD8+ (cytotoxic) T cells, which mediate target cell apoptosis by secreting perforin and granzyme or expressing Fas ligand, are the major players performing antitumor immune functions in HCC.26 However, they are always in a dysfunctional state characterized by impaired activity and proliferation, reduced cytokine production, and compromised cell-killing ability.27 Emerging investigations have shown that targeting miRNAs can improve the activity of CD8+ T cells and restore the state of immunosuppression in the TIME. MiR-206 promotes the recruitment of CD8+ T cells and prevents HCC by the M0-to-M1 transition of Kupffer cells driven by the activation of the KLF4/CCL2/CCR2 axis.28
CD4+ T Cells
CD4+ T cells are not only able to kill tumor cells directly but also exert an indirect role in the TIME as T helper cells. Certain CD4+ T cells directly lyse tumor cells.29 They not only cooperate with CD8+ T cells and macrophages to enhance antitumor effects but also differentiate into different subpopulations with different anti-tumor responses.30 MiR-26b-5p can promote the secretion of TNF-α, IFN-γ, IL-6, and IL-2 in CD4+ and CD8+ T cells by targeting PIM-2 in HCC, leading to enhanced T-cell responses.31 T helper cells (Th17) participate in many organ-specific autoimmune diseases. One report showed that the expression of miR-132 is higher in CD4+ IL-17+ cells than in CD4+ IL-17- cells. MiR-132 mediates the differentiation of Th17 cells and the secretion of IL-17 and IL-22, which increase the activation of hepatic stellate cells and strongly promote HCC migration and EMT.32 Regulatory T cells (Tregs) play an important role in maintaining self-tolerance and immune homeostasis and closely correlate with tumor progression, invasion, and metastasis.33 They can induce an immunosuppressive microenvironment by compromising immune surveillance against cancers and impairing antitumor immune responses. Accumulating evidence implies that miRNAs are considered key regulators in Tregs. Multiple reports have shown that miR-34a, miR-23a, miR-15a, and miR-16-1 play an important role in the regulation of Tregs.34–36
B Cells
Research on the immunobiology of HCC is of great significance for promoting the development of immunotherapy. There has been an exponential increase in research on the biological properties of T cells, but less on the role of B lymphocytes. Interestingly, B lymphocytes play a dual role in regulating tumor immunity.37 They can not only enhance humoral and cellular immunity but also promote tumor progression. One view put forward by Han is that the immune-related miRNAs harbored in exosomes may promote the differentiation of immunosuppressive B-cell subtypes in HCC.38 Hutter found that the miR-15a/16-1 and miR-15b/16-2 clusters limit progenitor B-cell proliferation by suppressing several prominent cell cycle regulators, such as Ccne1, Ccnd3, and Cdc25a, in vivo. In addition, loss of the miR-15 family (miR-15a/16-1 and miR-15b/16-2) in B-cell progenitors enhances IL7R expression and signaling which acts as a key regulator of early B-cells survival, proliferation, and differentiation.39 Therefore, it is difficult to determine their specific role,40 the regulatory effects and molecular mechanisms of miRNAs on B cells need to be further studied.
Natural Killer Cells
Natural killer (NK) cells are the first line of defense against cancers and virus infection by directly killing malignant cells without antibody involvement or MHC restriction. NK cells not only induce target cell apoptosis by releasing perforins and granzymes, expressing FasL, and mediating antibody-dependent cellular cytotoxicity (ADCC), but also directly act on target cells by secreting a large number of cytokines, such as ifn-v, tnf-x, gm-csf, il-3, and m-csf, or attack target cells.41 NK cells are beginning to become an attractive complement to T-cell-based immunotherapies based on the properties of independent antigen expression and rapid activation. Changes in the phenotype and function of NK cells have been described in patients with HCC, who also show perturbations of NK activating receptor/ligand axes.42
MiRNAs can enhance the cytotoxicity of NK cells. MiR-92b is a highly expressed microribonucleic acid in serum exosomes that improves the migration of liver cancer cell lines. Hepatoma-derived exosomal miR-92b may be transferred to NK cells, resulting in the downregulation of CD69 and weakening NK cell cytotoxicity.43 The target gene of miR-449c-5p is T-cell immunoglobulin and mucin domain 3 (TIM-3), which inhibits antitumoral immunity. HCC-derived exosomal circUHRF1 inhibits the cytotoxicity of NK cells by upregulating the expression of TIM-3 through degrading miR-449c-5p, leading to the promotion of immune evasion of HCC and resistance to anti-PD1 immunotherapy.44 The overexpression of miR-182 enhances the cytotoxicity of NK cells against tumorigenic Huh-7 cells by increasing the expression of activating receptor NKG2D and suppressing the receptor NKG2A.45 MiR-561-5p attenuates the antitumor response by downregulating CX3CL1 (a potential target of miR-561-5p) to reduce the infiltration and function of CX(3)CR1(+) NK cells.46
Tumor-Associated Macrophages in the TIME
Tumor-associated macrophages (TAMs), as the main type of inflammatory cells in the tumor microenvironment, exert either anti-tumorigenic (M1) or pro-tumorigenic (M2) functions.47 Monocytes differentiate into M1-like macrophages with antitumor properties under certain stimuli and signaling. However, M2-like macrophages can promote tumors, which promote tumor growth directly or indirectly, via the suppression of cytotoxic cell populations, including CD8+ T cells and NK cells.48 In the TIME, TAMs mainly show the properties of M2-type macrophages, leading to the promotion of tumor cell invasion, angiogenic switching, and immune escape of malignant cells.
Studies have reported the role of miRNAs in macrophage differentiation and polarization, which mediates the occurrence and progression of HCC. MiR-200b-3p exosomes are internalized by M0 macrophages and induce M2 polarization by down-regulating ZEB1 and up-regulating interleukin-4. Meanwhile, high levels of PIM1 and VEGFα expression are detected in M2 macrophages by activating the JAK/STAT signaling pathway, resulting in increased proliferation and metastasis of HCC.49 A study reported by Zhao elucidated that the expression of miR-144/miR-451a is regulated via chromatin remodeling dependent on DNA methylation. CpG island deletion of the miR-144/miR-451a promoter increases the expression of miR-144/miR-451a and reduces the expression of hepatocyte growth factor and macrophage migration inhibitory factor, leading to the promotion of M1-like polarization and the antitumor effect.50
Other Immune Cells
In addition to the immune cells mentioned above, miRNAs also play a role in regulating the biological function of other immune cells, such as tumor-associated neutrophils (TANs) and dendritic cells (DCs). TANs are neutrophils recruited into tumor tissues under the action of chemokines and involved in the genesis, development, and metastasis of tumors. They exhibit both antitumor (phenotype-N1) and protumor (phenotype-N2) activities in response to different stimuli.51 Ye pointed out that deregulated miR-223 participates in the pathogenesis of various liver diseases by influencing neutrophil infiltration, macrophage polarization, and inflammasome activation.52 The expression levels of CCL2 and CCL17 in TANs are significantly higher than neutrophils in peripheral blood. TANs contribute to HCC progression not only by recruiting macrophages and Treg cells but also by promoting the infiltration of macrophages and Treg cells through CCL2-CCR2 and CCL17-CCR4.53 Although a large number of studies have been conducted on miRNAs, none has adequately covered the mechanism by which miRNAs regulate immune cells in the TIME.
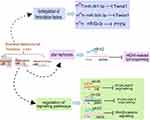
Regulation of the Angiogenesis
Under normal physiological conditions, angiogenesis is a highly ordered process regulated by complex and balanced interactions between pro-angiogenic and anti-angiogenic factors. With the rapid growth of tumors, the balance between proangiogenic and antiangiogenic factors is broken, and angiogenesis is subsequently activated. HCC is a solid tumor with a high degree of angiogenesis, these changes are advantageous to the growth, progression, invasion, and metastasis of HCC.54 Studies have revealed that miRNAs regulate tumor angiogenesis through different pathways (Figure 1). As early as 2013, Wang et al pointed out that miR-195 can directly inhibit VEGF levels and VEGF receptor 2 signaling in endothelial cells, leading to the suppression of angiogenesis and metastasis in HCC.55 MiR-375 affects antiangiogenesis by inhibiting platelet-derived growth factor C.56 MiR-200b-3p promotes angiogenesis by enhancing endothelial ERG (erythroblast transformation-specific (ETS)-related gene) expression.57
Cancer-associated endothelial cells (ECs) are essential for angiogenesis by affecting tube formation. MiRNAs play an important role in this process. MiR-210 directly targets SMAD4 and STAT6 and inhibits their expression to stimulate EC tubulogenesis, leading to the promotion of angiogenesis.58 Exosomal miR-638 from HuH-7Mb decreases the expression of VE-cadherin and ZO-1, resulting in attenuating endothelial tight junctions and increasing the permeability of FITC-dextran.59 Nevertheless, the role of antiangiogenic agents in cancers is disappointing, partly because the precise molecular mechanisms of angiogenesis are unknown and partly because antiangiogenic agents may restrict drug delivery to the tumor, resulting in reduced clinical efficacy.60
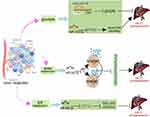
Regulation of EMT
Epithelial-mesenchymal transition (EMT) is a complex phenotypic event characterized by the morphologic transformation of cells from an epithelioid to a mesenchymal appearance, which plays an important role in embryogenesis, stem cell biology, and cancer progression. Studies have shown that miRNAs regulate the EMT by affecting different transcription factors and signaling pathways (Figure 2).
MiR-509-3p inhibits EMT by targeting Twist which is a critical inducible transcription factor for EMT.61 There is a growing body of reports suggesting that miRNAs affect EMT by regulating signaling pathways. miR-92a-3p is involved in the regulation of EMT by inhibiting the PTEN/Akt/Snail pathways.62 MiR-300 inhibits the EMT by targeting FAK and the downstream PI3K/AKT pathway, leading to reduced migration and invasion of HCC.63
In addition to these, studies have shown that miRNAs participate in the regulation of EMT by interacting with other ncRNAs, such as lncRNAs, and circRNAs. CircTOLLIP serves as a sponge for miR-516a-5p to alleviate its inhibition of PBX3, leading to the promotion of EMT and the progression in HCC.64 Similar results were found that lncRNA SNHG12 can upregulate HEG1 by targeting and inhibiting miR-516a-5p, resulting in the promotion of EMT in HCC.65
Furthermore, miRNAs are involved in the EMT by regulating metabolic pathways, which has received increasing attention. MiR-612 inhibits the EMT by directly targeting HADHA which promotes β-oxidation of fatty acids in HCC.66 MiR-186 inhibits the EMT, migration, and invasion in HepG2 and HUH7 cells by directly targeting cyclin-dependent kinase 6 (one of the cell cycle-related genes).67
However, it is difficult to obtain the same results, due to the molecular heterogeneity of HCC as well as different experimental approaches and control groups selected by laboratories, even though contrary results may be obtained in some cases. The regulatory functions and underlying mechanisms of miRNAs in EMT need further investigation.
Regulation of Tumor Invasion and Metastasis
HCC is always accompanied by intrahepatic vascular microinvasion and micrometastasis at an early stage, resulting in high aggression and poor prognosis, with a recurrence rate of over 70% in 5 years. Therefore, the molecular mechanism of invasion and metastasis in HCC urgently needs further studies. Research about miRNAs on the complex invasive-metastatic cascade response provides new insights into the HCC treatment.
On the one hand, miRNAs play a role in promoting tumor invasion and metastasis in HCC. A-kinase anchor protein 12 (AKAP12), as a scaffolding protein in signal transduction, exerts an antitumor role in various cancers including HCC. MiR-1251-5p promotes the migration and invasion of HCC in vitro by directly targeting and negatively regulating the expression of AKAP12.68 On the other hand, miRNAs exert an inhibitory effect on the invasion and metastasis of HCC. MiR-7 can inhibit the invasion and metastasis of hepatoma cells by downregulating EMT-related proteins.69 MiR-145 suppresses HCC metastasis and invasion by directly targeting ARF6 and inhibiting its expression.70
In addition, scientists have explored how miRNAs regulate the invasion and metastasis in HCC from new perspectives. Chronic inflammatory stimuli are indispensable in tumorigenesis and progression such as IL-8, IL-6, and IL-10. Peng found that IL-8 negatively modulates the expression of miR370-3p by recruiting histone deacetylase 1 (HDAC1) to the miR-370-3p promoter. MiR-370-3p attenuates IL-8 protumoral effects on liver cancer cells by directly targeting Snail and Twist1. The novel axis IL-8/STAT3/miR-370-3p/Twist1 and Snail relying on HDAC1 recruitment provides new insights into the diagnosis and treatment of HCC.71 Studies have also shown that abnormal lipid metabolism is involved in the regulation of miRNAs on the invasion and metastasis of tumors. The miR-377-3p/CPT-1C axis regulates the proliferation, migration, and invasion of HCC mainly through continuous fatty acid oxidation (FAO), which is essential for cancers to produce extra energy to maintain the rapid proliferation of cells.72
The invasion and metastasis in HCC are complex processes involving multiple factors, the regulatory functions and underlying mechanisms of miRNAs in these processes have yet to be fully elucidated. More in-depth research is needed in the future.
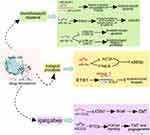
Regulation of Tumor Metabolism
To accommodate the insufficient supply of nutrients and oxygen, metabolic reprogramming becomes an essential survival strategy for tumors. The most classic event of metabolic reprogramming in cancer is the Warburg effect, also known as aerobic glycolysis, which is considered a hallmark of cancers and an indicator of poor prognosis. Even so, malignant cells prefer aerobic glycolysis in case of sufficient oxygen.18
Studies have revealed that miRNAs are involved in regulating the aerobic glycolysis. Forkhead box K1 (FOXK1) is a transcription factor that promotes the progression of multiple cancers. Xing et al reported that miR-144-3p inhibits glycolysis by targeting FOXK1, thereby inhibiting the malignant progression of HCC.73 MiR-183-5p could increase aerobic glycolysis by targeting PTEN and then activating Akt/mTOR signaling, leading to the promotion of migration and invasion in HCC.74
The Warburg effect results in a large production and accumulation of lactate, which enhances the immunosuppressive properties of the TIME. Komoll et al found that miR-342-3p plays a suppressor role in HCC by targeting and modulating the lactate transport function of member 1/ monocarboxylate transporter 1 (SLC16A1/MCT1). Mechanistically, the downregulation of MCT1 expression by miR-342-3p impairs lactate transport, resulting in an increase in extracellular lactate level and a decrease in intracellular lactate level. This change attenuated immunosuppression, resulting in a significant suppression of tumor progression and prolonged survival in HCC patients.75 In addition to participating in glycolytic and lactate metabolism, miR-377-3p and miR-21 play important roles in hepatic lipid metabolism.72,76
Amino acid metabolic remodeling is another important factor affecting the malignant activities of cancers. MiR-122 is involved in Gln metabolism and transport in hepatic cells by inhibiting the expression of GLS (Gln metabolism) and SLC1A5 (Gln transport). HCC patients with higher levels of GLS and SLC1A5 have a significantly lower survival rate than patients with lower levels.77 TDO2 (tryptophan 2,3-dioxygenase), is highly expressed in HCC and involved in immune tolerance. MiR-126-5p affects cell proliferation and metastasis by directly targeting and promoting the expression of TDO2.78 Targeting tumor metabolic pathways may become an effective therapeutic strategy for HCC treatment. The underlying mechanisms of miRNAs in regulating tumor metabolism (Figure 3) provide new insights into HCC treatment.
Regulation of Drug Resistance
With the advent of molecularly targeted drugs and immune checkpoint inhibitors (ICIs), a breakthrough has been made in HCC treatment. Nonetheless, treatment resistance has become one of the major barriers to the failure of HCC treatment, and more than 90% of cancer patients’ deaths are associated with chemotherapy resistance.79 Studies have shown that miRNAs play important roles in drug resistance by fine-tuning key physiological and pathophysiological processes.
Sorafenib, a kinase inhibitor, is commonly used in the treatment of patients with advanced HCC. MiR-124-3p.1 enhances sorafenib-induced apoptosis by affecting the acetylation and nuclear localization of FOXO3a by targeting SIRT1 and AKT2, respectively.80 MiR-138-1-3p directly targets PAK5 and inhibits its expression, up-regulation of PAK5 contributes to the sorafenib resistance of HCC via β-catenin/ABCB1 signaling pathway.81 Lenvatinib is another first-line multikinase inhibitor for patients with advanced HCC. MiR-128-3p regulates lenvatinib resistance through proliferation and apoptosis-related signaling pathways by downregulating c-Met.82
EMT-associated miRNAs play a crucial role in drug resistance. MiR-125b-5p is involved in sorafenib resistance by inducing EMT through up-regulating the Snail.83 However, miR-541 increases the sensitivity of HCC cells to sorafenib treatment by directly targeting and down-regulating autophagy-related gene 2A (ATG2A).84 Besides, miRNAs regulate drug resistance through the ferroptosis. The overexpression of miR-23a-3p attenuates sorafenib-induced ferroptosis by targeting ACSL4, resulting in sorafenib resistance.85
The mechanisms of miRNAs in regulating drug resistance are being investigated in depth (Figure 4). However, it is difficult to fully elucidate the mechanism of miRNAs in regulating drug resistance because it is the result of the synergistic effect of multiple genes and mechanisms.
Clinical Application of MiRNAs in HCC
Being diagnosed at an advanced stage and a high recurrence rate are leading causes of the poor prognosis of HCC. Looking for diagnostic/prognostic biomarkers, especially in the early stage, is significant for the prevention and treatment of HCC. The pathological diagnosis of HCC is the “gold standard”, but its clinical application should be very cautious because it is an invasive approach and has a risk of metastasizing. However, traditional biomarkers have shown poor performance in the monitoring, diagnosis, and prognosis of HCC. Circulating miRNAs are secreted into extracellular spaces and extremely stable in biological fluids (eg, serum, plasma, and urine), their changes indicate the status of cancers and the prognosis of patients. Accumulating studies have shown that circulating miRNAs have potential clinical application in the diagnosis and prognosis of HCC as minimally invasive biomarkers. This section summarizes the main diagnostic/prognostic indicators which are currently being studied.
MiRNAs Act as Diagnostic Biomarkers
The utility of circulating miRNAs as diagnostic markers for HCC is evaluated through a clinical trial database or clinical trials. More details are shown in Table 1. As early as 2015, it was reported that miR-21 can be used as an early diagnostic marker for HCC, the change of serum miR-21 occurs earlier and more accurately reflects the pathogenesis of HCC than the change of AFP.86 Studies have shown that both plasma and urine miR-39-5p can be used to detect early, advanced, and overall HBV-associated HCC cases with more than 85% sensitivities and 93% specificities. Interestingly, miR-93-5p in urine could be used to predict the prognosis of patients with HBV-related HCC.87 Emerging investigations demonstrate that developing a platform of multiple miRNAs could improve their sensitivity and specificity for HCC detection. A combination of these three miRNAs (miR-122-5p, let-7d-5p, and miR-425-5p) improves the accuracy of diagnoses for HCC, with an area under the curve (AUC) of 0.97.88 The exosomal miRNA panel containing miRNA-122, miRNA-21, and miRNA-96 has high accuracy in discriminating HCC from the cirrhosis group and healthy volunteers’ group with a higher AUC value of 0.924.89 A view that the combination of miRNAs with AFP may be a more desirable diagnostic modality. Li reported that the combined detection of serum miR-221 and AFP had a sensitivity of 96.49% and an accuracy of 93.10% for HCC.90 Clinical trials of miR-21 and miR-221 as early diagnostic markers for HCC have been conducted, and the details can be found by querying NCT05449847 and NCT02928627, respectively.
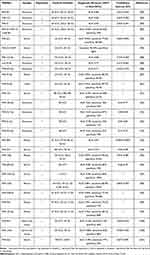 |
Table 1 MiRNAs as Diagnostic Biomarkers for HCC |
MiRNAs Act as Prognostic Biomarkers
Searching for specific biomarkers of the prognosis is crucial to HCC treatment. Abnormal expression and regulation of miRNAs in serum/plasma, especially in exosomes, is regarded as a “beacon” of cancer prognosis.
Lee pointed out that the overall survival and progression-free survival are significantly lower in HCC patients with higher level of exosomal miRNA-21 (≥0.09) (Log rank test: p<0.05). MiRNA-21 is associated not only with TNM staging but also with T staging and portal vein thrombosis.102 Another study has shown that a low expression of miR-33b is strongly associated with tumor volume, metastasis, and higher clinical stage of HCC.103 Studies show that abnormal expressions of miR-637, miRNA-29a, miR-21, and miR-122 could be used as promising prognostic markers for HCC.104–106 Details are shown in Table 2.
 |
Table 2 MiRNAs as Prognostic Biomarkers for HCC |
Therapeutic Potential of miRNAs in HCC
For a long time, Proteins have been widely considered to be the targets of most drugs in human diseases, and many new drugs targeting them have been introduced.114,115 Studies have shown that most proteins are “nondruggable”, only 0.2% of the genome codes for disease-related proteins.116 Therefore, the feasibility of targeting RNA has attracted increasing attention. MiRNAs are involved in the occurrence and development of cancers. The regulatory mechanisms of miRNAs are delicate and complex, one miRNA may have multiple target genes, and one gene may be regulated by several miRNAs. So, miRNAs are at the core of a large and fine regulatory network. In this section, we will summarize the research progress of miRNAs in the treatment of HCC, especially its precision therapy.
There are two main therapeutic strategies for miRNAs: replacement, restoration, or overexpression therapy, such as miRNA mimics, and miRNA-targeted therapeutics, including miRNA reduction, inhibition, or downregulation, such as antagomiRs.117 MiRNA replacement therapeutics are achieved by importing chemically synthesized miRNAs or miRNA mimics into hepatocytes to restore/enhance tumor suppressor miRNAs.118 A report conducted by Komoll indicated that administration of adeno-associated virus vector (AAV)-mediated miR-342-3p (AAV-miR-342-3p) can significantly attenuate tumor development and prolong overall survival In different mouse models of HCC.75 Intravenously injected liposome-based miR-34 (MRX34) is the first-in-class miRNA replacement therapy for patients with advanced HCC. MiR-34a plays a vital role in inhibiting the progression of HCC by suppressing the expression of hexokinase-1, TRAF5, E2F1, and E2F3. Its Phase I trial was initiated in May 2013. Although the preliminary results promised a tolerable safety profile for patients with advanced HCC, several patients died during MRX34 replacement therapy owing to serious immune-related adverse events.119 Similar findings were shown in another study.120 Now, the development of MRX34 has been halted. However, whether miR-34a has the specific gene-suppressing activity, the double-stranded RNA (dsRNA) of the MRX34 has the nonspecific inflammatory effect or the effective delivery of these RNA constructs affects the clinical effects (both toxicity and antitumor activity) remains to be further studied.
MiRNA-targeted therapeutics are achieved by regulating the expression of oncogenic miRNAs with specific miRNA inhibitors. Miravirsen, against miR-122, is the first miRNA inhibitor. The Phase II clinical trial of Miravirsen for the treatment of hepatitis C virus was successfully completed.121 AC1MMYR2, a small-molecule inhibitor of miR-21, reverses EMT and inhibits tumor growth, invasion, and metastasis by blocking miR-21 maturation without significant tissue cytotoxicity.122 What excited us is that a study from Hassan holds much promise for the miR-122 mimic/miR-221 inhibitor combination as an innovative therapeutic strategy for HCC in a mouse model induced by DEN. Combined treatment with miR-122 mimic /miR-221 antagonist is the most effective technique compared to treatment with miR-122 mimic or miR-221 inhibitor alone. Mechanistically, coadministration of miR-122 mimic and miR-221 inhibitor dramatically downregulates the expression of cyclin D1, TGF-β, and β-catenin genes which play significant roles in the regulation of the cell cycle, EMT, and cell proliferation, respectively.123
With the rapid development of sequencing technologies and significant advances in miRNA research, miRNA therapeutics remain the most promising treatments for tumors, although they are still in the experimental stage.
Conclusion
In this review, we focus on the roles of miRNAs in regulating TIME, angiogenesis, EMT, invasion, metastasis, metabolism, and drug resistance and their molecular mechanisms in HCC. MiRNAs are not only large in number but also complex in function, one miRNA may have multiple target genes, or several miRNAs may simultaneously target and regulate one gene. Whether the dysregulation of miRNAs is a cause or a consequence of cancers has not been fully elucidated, and more in-depth studies are needed in the future. In addition, we provide a comprehensive overview of the roles and promising approaches of miRNAs in the diagnosis and therapy of HCC. However, there are no miRNA therapies for HCC approved by the FDA yet. The main reason is that miRNA therapies for HCC are still in the experimental stage, and there are still many challenges to be solved. One challenge is that the roles and underlying molecular mechanisms of miRNAs in HCC have not been fully elucidated. Another challenge is the toxicity of miRNAs in patients, such as severe immune responses and off-target effects on the other genes. Facing the challenges, the following strategies are needed. First, the next-generation sequencing technology is vital to explore the vast uncharted territory of miRNAs and their emerging roles. Studying the function of miRNAs in clinical Settings is as important as at the cellular or animal level. Second, improving targeting methods and delivery systems of therapeutic miRNAs can minimize the immune responses and overcome the off-target effects. Finally, interdisciplinary cooperation in several fields such as immunology, molecular biology, pharmacology, and nanotechnology can promote the development of HCC therapeutic strategies based on miRNAs. Nevertheless, it remains a focus of attention in the pharmaceutical industry and will be a promising tool for personalized therapy alone or in combination with other therapies shortly.
Acknowledgments
First of all, I would like to thank Professor Zong Jinbao, whose guidance and suggestions in the field of hepatocellular carcinoma have greatly helped me to complete this paper. Secondly, I would like to thank the other authors who generously helped me to collect information and gave me many valuable suggestions.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sung H, Ferlay J, Siegel RL. et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi:10.3322/caac.21660
2. Philips CA, Rajesh S, Nair DC, et al. Hepatocellular Carcinoma in 2021: an Exhaustive Update. Cureus. 2021;13(11):e19274. doi:10.7759/cureus.19274
3. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
4. Chen H, Cheng H, Dai Q, et al. A superstable homogeneous lipiodol-ICG formulation for locoregional hepatocellular carcinoma treatment. J Control Release. 2020;323:635–643. doi:10.1016/j.jconrel.2020.04.021
5. Liang X, Liu H Chen H, et al. Rhein-based Pickering emulsion for hepatocellular carcinoma: shaping the metabolic signaling and immunoactivation in transarterial chemoembolization. Aggregate. 2022;2024:e552
6. Bayraktar E, Bayraktar R, Oztatlici H, et al. Targeting miRNAs and Other Non-Coding RNAs as a Therapeutic Approach: an Update. Noncoding RNA. 2023;9(2):27.
7. Wu J, Liu W, Qiu X, et al. A Noninvasive Approach to Evaluate Tumor Immune Microenvironment and Predict Outcomes in Hepatocellular Carcinoma. Phenomics. 2023;3(6):549–564. doi:10.1007/s43657-023-00136-8
8. Guo BJ, Ruan Y, Wang YJ, et al. Jiedu Recipe, a compound Chinese herbal medicine, inhibits cancer stemness in hepatocellular carcinoma via Wnt/beta-catenin pathway under hypoxia. J Integr Med. 2023;21(5):474–486. doi:10.1016/j.joim.2023.06.008
9. Huang A, Yang XR, Chung WY, et al. Targeted therapy for hepatocellular carcinoma. Signal Transduct Target Ther. 2020;5(1):146. doi:10.1038/s41392-020-00264-x
10. Offringa R, Kötzner L, Huck B, et al. The expanding role for small molecules in immuno-oncology. Nat Rev Drug Discov. 2022;21(11):821–840. doi:10.1038/s41573-022-00538-9
11. Ilyas FZ, Beane JD, Pawlik TM. The State of Immunotherapy in Hepatobiliary Cancers. Cells. 2021;10(8):2096. doi:10.3390/cells10082096
12. Baharom F, Ramirez-Valdez RA, Khalilnezhad A, et al. Systemic vaccination induces CD8+ T cells and remodels the tumor microenvironment. Cell. 2022;185(23):4317–4332. doi:10.1016/j.cell.2022.10.006
13. Oura K, Morishita A, Tani J, et al. Tumor immune microenvironment and immunosuppressive therapy in hepatocellular carcinoma: a review. Int J Mol Sci. 2021;22(11):5801. doi:10.3390/ijms22115801
14. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. 2020;77:1745–1770.
15. Lopes-Coelho F, Martins F, Pereira SA, et al. Anti-Angiogenic Therapy: current Challenges and Future Perspectives. Int J Mol Sci. 2021;22(7):3765. doi:10.3390/ijms22073765
16. Brabletz S, Schuhwerk H, Brabletz T, et al. Dynamic EMT: a multi-tool for tumor progression. EMBO J. 2021;40(18):e108647. doi:10.15252/embj.2021108647
17. Lin YL, Li Y. Study on the hepatocellular carcinoma model with metastasis. Genes Dis. 2020;7(3):336–350. doi:10.1016/j.gendis.2019.12.008
18. Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: still emerging. Cell Metab. 2022;34(3):355–377. doi:10.1016/j.cmet.2022.01.007
19. Martínez-Reyes I, Chandel NS. Cancer metabolism: looking forward. Nat Rev Cancer. 2021;21(10):669–680. doi:10.1038/s41568-021-00378-6
20. Liang Y, Liang Q, Qiao L, et al. MicroRNAs modulate drug resistance-related mechanisms in hepatocellular carcinoma. Front Oncol. 2020;10:920. doi:10.3389/fonc.2020.00920
21. Budakoti M, Panwar AS, Molpa D, et al. Micro-RNA: the darkhorse of cancer. Cell Signal. 2021;83:109995. doi:10.1016/j.cellsig.2021.109995
22. Tavakoli Pirzaman A, Alishah A, Babajani B, et al. The Role of microRNAs in Hepatocellular Cancer: a Narrative Review Focused on Tumor Microenvironment and Drug Resistance. Technol Cancer Res Treat. 2024;23:15330338241239188. doi:10.1177/15330338241239188
23. Gramantieri L, Giovannini C, Piscaglia F, et al. MicroRNAs as Modulators of Tumor Metabolism, Microenvironment, and Immune Response in Hepatocellular Carcinoma. J Hepatocell Carcinoma. 2021;8:369–385. doi:10.2147/JHC.S268292
24. Fan X, Ke L, Cheng H, et al. Enhanced drug retention by anthracene crosslinked nanocomposites for bimodal imaging-guided phototherapy. Nanoscale. 2021;13(35):14713–14722. doi:10.1039/D1NR04171A
25. Xu S, Cai J, Cheng H, et al. Sustained release of therapeutic gene by injectable hydrogel for hepatocellular carcinoma. Int J Pharm. 2023;6:100195.
26. Zhao Q, Wongpoomchai R, Chariyakornkul A, et al. Identification of gene-set signature in early-stage hepatocellular carcinoma and relevant immune characteristics. Front Oncol. 2021;11:740484. doi:10.3389/fonc.2021.740484
27. Dolina JS, Van Braeckel-Budimir N, Thomas GD, et al. CD8+ T Cell Exhaustion in Cancer. Front Immunol. 2021;12:715234. doi:10.3389/fimmu.2021.715234
28. Liu N, Wang X, Steer CJ, et al. MicroRNA-206 promotes the recruitment of CD8+ T cells by driving M1 polarisation of Kupffer cells. Gut. 2022;71(8):1642–1655. doi:10.1136/gutjnl-2021-324170
29. Quezada SA, Simpson TR, Peggs KS, et al. Tumor-reactive CD4(+)T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi:10.1084/jem.20091918
30. Accogli T, Bruchard M, Végran F. Modulation of CD4 T cell response according to tumor cytokine microenvironment. Cancers. 2021;13(3):37. doi:10.3390/cancers13030373
31. Han W, Li N, Liu J, et al. MicroRNA-26b-5p enhances T cell responses by targeting PIM-2 in hepatocellular carcinoma. Cell Signal. 2019;59:182–190. doi:10.1016/j.cellsig.2018.11.011
32. Feng R, Cui Z, Liu Z, et al. Upregulated microRNA-132 in T helper 17 cells activates hepatic stellate cells to promote hepatocellular carcinoma cell migration in vitro. Scand J Immunol. 2021;93(e13007). doi:10.1111/sji.13007.
33. Nishikawa H, Koyama S. Mechanisms of regulatory T cell infiltration in tumors: implications for innovative immune precision therapies. J Immunother Cancer. 2021;9(7):e002591. doi:10.1136/jitc-2021-002591
34. Yang P, Li QJ, Feng Y, et al. TGF-β-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22(3):291–303. doi:10.1016/j.ccr.2012.07.023
35. Li ZQ, Wang HY, Zeng QL, et al. p65/miR-23a/CCL22 axis regulated regulatory T cells recruitment in hepatitis B virus positive hepatocellular carcinoma. Cancer Med. 2020;9(2):711–723. doi:10.1002/cam4.2611
36. Li A, Shuai X, Jia Z, et al. Ganoderma lucidum polysaccharide extract inhibits hepatocellular carcinoma growth by downregulating regulatory T cells accumulation and function by inducing microRNA-125b. J Transl Med. 2015;13(1):100. doi:10.1186/s12967-015-0465-5
37. Michaud D, Steward CR, Mirlekar B, et al. Regulatory B cells in cancer. Immunol Rev. 2021;299(1):74–92. doi:10.1111/imr.12939
38. Han Q, Zhao H, Jiang Y, et al. HCC-Derived Exosomes:Critical Player and Target for Cancer Immune Escape. Cells. 2019;8(6):558. doi:10.3390/cells8060558
39. Hutter K, Rülicke T, Szabo TG, et al. The miR-15a/16-1 and miR-15b/16-2 clusters regulate early B cell development by limiting IL-7 receptor expression. Front Immunol. 2022;13:967914. doi:10.3389/fimmu.2022.967914
40. Engelhard V, Conejo-Garcia JR, Ahmed R, et al. B cells and cancer. Cancer Cell. 2021;39(10):1293–1296. doi:10.1016/j.ccell.2021.09.007
41. Cózar B, Greppi M, Carpentier S, et al. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021;11(1):34–44. doi:10.1158/2159-8290.CD-20-0655
42. Mantovani S, Oliviero B, Varchetta S, et al. Natural killer cell responses in hepatocellular carcinoma: implications for novel immunotherapeutic approaches. Cancers. 2020;12(4):926. doi:10.3390/cancers12040926
43. Nakano T, Chen IH, Wang CC, et al. Circulating exosomal miR-92b: its role for cancer immunoediting and clinical value for prediction of posttransplant hepatocellular carcinoma recurrence. Am J Transplant. 2019;19(12):3250–3262. doi:10.1111/ajt.15490
44. Zhang PF, Gao C, Huang XY, et al. Cancer cell-derived exosomal circUHRF1 induces natural killer cell exhaustion and may cause resistance to anti-PD1 therapy in hepatocellular carcinoma. Mol Cancer. 2020;19(1):110. doi:10.1186/s12943-020-01222-5
45. Abdelrahman MM, Fawzy IO, Bassiouni AA, et al. Enhancing NK cell cytotoxicity by miR-182 in hepatocellular carcinoma. Hum Immunol. 2016;77(8):667–673. doi:10.1016/j.humimm.2016.04.020
46. Chen EB, Zhou ZJ, Xiao K, et al. The miR-561-5p/CX 3 CL1 Signaling Axis Regulates Pulmonary Metastasis in Hepatocellular Carcinoma Involving CX 3 CR1+ Natural Killer Cells Infiltration. Theranostics. 2019;9(16):4779–4794. doi:10.7150/thno.32543
47. Lecoultre M, Dutoit V, Walker PR. Phagocytic function of tumor-associated macrophages as a key determinant of tumor progression control: a review. J Immunother Cancer. 2020;8(2):e001408. doi:10.1136/jitc-2020-001408
48. Arvanitakis K, Koletsa T, Mitroulis I, et al. Tumor-Associated Macrophages in Hepatocellular Carcinoma Pathogenesis, Prognosis and Therapy. Cancers. 2022;14(1):226. doi:10.3390/cancers14010226
49. Xu Y, Luan G, Liu F, et al. Exosomal miR-200b-3p induce macrophage polarization by regulating transcriptional repressor ZEB1 in hepatocellular carcinoma. Hepatol Int. 2023;17(4):889–903. doi:10.1007/s12072-023-10507-y
50. Zhao J, Li H, Zhao S, et al. Epigenetic silencing of miR-144/451a cluster contributes to HCC progression via paracrine HGF/MIF-mediated TAM remodeling. Mol Cancer. 2021;20(1):46. doi:10.1186/s12943-021-01343-5
51. Zhao Y, Rahmy S, Liu Z, et al. Rational targeting of immunosuppressive neutrophils in cancer. Pharmacol Ther. 2020;212:107556. doi:10.1016/j.pharmthera.2020.107556
52. Ye D, Zhang T, Lou G, et al. Role of miR-223 in the Pathophysiology of liver diseases. Exp Mol Med. 2018;50(9):1–12. doi:10.1038/s12276-018-0153-7
53. Zhou SL, Zhou ZJ, Hu ZQ, et al. Tumor-Associated Neutrophils Recruit Macrophages and T-Regulatory Cells to Promote Progression of Hepatocellular Carcinoma and Resistance to Sorafenib. Gastroenterology. 2016;150(1646):1658.e17. doi:10.1053/j.gastro.2016.02.040
54. Yao C, Wu S, Kong J, et al. Angiogenesis in hepatocellular carcinoma: mechanisms and anti-angiogenic therapies. Cancer Biol Med. 2023;20(1):25–43. doi:10.20892/j.issn.2095-3941.2022.0449
55. Wang R, Zhao N, Li S, et al. MicroRNA-195 suppresses angiogenesis and metastasis of VEGF, VAV2, and CDC42. Hepatology. 2013;58(2):642–653. doi:10.1002/hep.26373
56. Li D, Wang T, Sun FF, et al. MicroRNA-375 represses tumor angiogenesis and reverses resistance to sorafenib in hepatocarcinoma. Cancer Gene Ther. 2021;28(1–2):126–140. doi:10.1038/s41417-020-0191-x
57. Moh-Moh-Aung A, Fujisawa M, Ito S, et al. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. Sci Rep. 2020;10(1):10418. doi:10.1038/s41598-020-67425-4
58. Lin XJ, Fang JH, Yang XJ, et al. Hepatocellular Carcinoma Cell-Secreted Exosomal MicroRNA-210 Promotes Angiogenesis In Vitro and In Vivo. Mol Ther Nucleic Acids. 2018;11:243–252. doi:10.1016/j.omtn.2018.02.014
59. Yokota Y, Noda T, Okumura Y, et al. Serum exosomal miR-638 is a prognostic marker of HCC via downregulation of VE-cadherin and ZO-1 of endothelial cells. Cancer Sci. 2021;112(3):1275–1288. doi:10.1111/cas.14807
60. Dumas SJ, García-Caballero M, Carmeliet P. Metabolic Signatures of Distinct Endothelial Phenotypes. Trends Endocrinol Metab. 2020;31(8):580–595. doi:10.1016/j.tem.2020.05.009
61. Zhang H, Liu S, Chen L, et al. MicroRNA miR-509-3p inhibit metastasis and epithelial-mesenchymal transition in hepatocellular carcinoma. Bioengineered. 2021;12(1):2263–2273. doi:10.1080/21655979.2021.1932210
62. Yang B, Feng X, Liu H, et al. High-metastatic cancer cells derived exosomal miR92a-3p promotes epithelial-mesenchymal transition and metastasis of low-metastatic cancer cells by regulating PTEN/Akt pathway in hepatocellular carcinoma. Oncogene. 2020;39(42):6529–6543. doi:10.1038/s41388-020-01450-5
63. Wang R, Yu Z, Chen F, et al. miR-300 regulates the epithelial-mesenchymal transition and invasion of hepatocellular carcinoma by targeting the FAK/PI3K/AKT signaling pathway. Biomed Pharmacother. 2018;103(1632):1642. doi:10.1016/j.biopha.2018.03.005
64. Liu Y, Song J, Zhang H, et al. EIF4A3-induced circTOLLIP promotes the progression of hepatocellular carcinoma via the miR-516a-5p/PBX3/EMT pathway. J Exp Clin Cancer Res. 2022;41(1):164. doi:10.1186/s13046-022-02378-2
65. Chen PP, Zhang ZS, Wu JC, et al. LncRNA SNHG12 promotes proliferation and epithelial mesenchymal transition in hepatocellular carcinoma through targeting HEG1 via miR-516a-5p. Cell Signal. 2021;84:109992. doi:10.1016/j.cellsig.2021.109992
66. Liu Y, Lu LL, Wen D, et al. MiR-612 regulates invadopodia of hepatocellular carcinoma by HADHA-mediated lipid reprogramming. J Hematol Oncol. 2020;13(1):44. doi:10.1186/s13045-020-00875-5
67. Lu J, Zhao Z, Ma Y. miR-186 represses proliferation, migration, invasion, and EMT of hepatocellular carcinoma via directly targeting CDK6. Oncol Res. 2020;28(5):509–518. doi:10.3727/096504020X15954139263808
68. Han S, Wang L, Sun L, et al. MicroRNA-1251-5p promotes tumor growth and metastasis of hepatocellular carcinoma by targeting AKAP12. Biomed Pharmacother. 2020;122:109754. doi:10.1016/j.biopha.2019.109754
69. Yuan J, Li Y, Liao J, et al. MicroRNA-7 inhibits hepatocellular carcinoma cell invasion and metastasis by regulating Atg5-mediated autophagy. Transl Cancer Res. 2020;9(6):3965–3972. doi:10.21037/tcr-20-1930
70. Wang S, Wang T, Gu P. microRNA-145-5p Inhibits Migration, Invasion, and Metastasis in Hepatocellular Carcinoma by Inhibiting ARF6. Cancer Manag Res. 2021;13:3473–3484. doi:10.2147/CMAR.S300678
71. Peng S, Chen Y, Li T, et al. Hsa-microRNA-370-3p targeting Snail and Twist1 suppresses IL-8/STAT3-driven hepatocellular carcinoma metastasis. Cancer Sci. 2022;113:4120–4134. doi:10.1111/cas.15571
72. Zhang T, Zhang Y, Liu J, et al. MicroRNA-377-3p inhibits hepatocellular carcinoma growth and metastasis through negative regulation of CPT1C-mediated fatty acid oxidation. Cancer Metab. 2022;10:2.
73. Xing B, Shen C, Yang Q, et al. R-144-3p represses hepatocellular carcinoma progression by affecting cell aerobic glycolysis via FOXK1. Int J Exp Pathol. 2023;104(3):117–127. doi:10.1111/iep.12468
74. Niu Y, Liu F, Wang X, et al. miR-183-5p Promotes HCC Migration/Invasion via Increasing Aerobic Glycolysis. Onco Targets Ther. 2021;14:3649–3658. doi:10.2147/OTT.S304117
75. Komoll RM, Hu Q, Olarewaju O, et al. MicroRNA-342-3p is a potent tumour suppressor in hepatocellular carcinoma. J Hepatol. 2021;74(1):122–134. doi:10.1016/j.jhep.2020.07.039
76. Lai CY, Yeh KY, Lin CY, et al. MicroRNA-21 plays multiple oncometabolic roles in the process of NAFLD-related hepatocellular carcinoma via PI3K/AKT, TGF-b, and STAT3 signaling. Cancers. 2021;13(5):940. doi:10.3390/cancers13050940
77. Sengupta D, Cassel T, Teng KY, et al. Regulation of hepatic glutamine metabolism by miR-122. Mol Metab. 2020;34:174–186. doi:10.1016/j.molmet.2020.01.003
78. Ai Y, Luo S, Wang B, et al. MiR-126-5p Promotes Tumor Cell Proliferation, Metastasis and Invasion by Targeting TDO2 in Hepatocellular Carcinoma. Molecules. 2022;27(2):443. doi:10.3390/molecules27020443
79. Ajith AK, Subramani S, Manickam AH, et al. Chemotherapeutic Resistance Genes of Breast Cancer Patients-An Overview. Adv Pharm Bull. 2022;12(4):649–657. doi:10.34172/apb.2022.048
80. Dong ZB, Wu HM, He YC, et al. MiRNA-124-3p.1 sensitizes hepatocellular carcinoma cells to sorafenib by regulating FOXO3a by targeting AKT2 and SIRT1. Cell Death Dis. 2022;13(1):35. doi:10.1038/s41419-021-04491-0
81. Li TT, Mou J, Pan YJ, et al. MicroRNA-138-1-3p sensitizes sorafenib to hepatocellular carcinoma by targeting PAK5 mediated β-catenin/ABCB1 signaling pathway. J Biomed Sci. 2021;28(1):56. doi:10.1186/s12929-021-00752-4
82. Xu X, Jiang W, Han P, et al. MicroRNA-128-3p Mediates Lenvatinib Resistance of Hepatocellular Carcinoma Cells by Downregulating c-Met. J Hepatocell Carcinoma. 2022;27(9):113–126. doi:10.2147/JHC.S349369
83. Hirao A, Sato Y, Tanaka H, et al. MiR-125b-5p Is Involved in Sorafenib Resistance through Ataxin-1-Mediated Epithelial Mesenchymal Transition in Hepatocellular Carcinoma. Cancers. 2021;13(19):4917. doi:10.3390/cancers13194917
84. Xu WP, Liu JP, Feng JF, et al. MiR-541 potentiates the response of human hepatocellular carcinoma to sorafenib treatment by inhibiting autophagy. Gut. 2020;69(7):1309–1321. doi:10.1136/gutjnl-2019-318830
85. Lu Y, Chan YT, Tan HY, et al. Epigenetic regulation of ferroptosis via ETS1/miR-23a-3p/ACSL4 axis mediates sorafenib resistance in human hepatocellular carcinoma. J Exp Clin Cancer Res. 2022;41(1):3. doi:10.1186/s13046-021-02208-x
86. Wang X, Zhang J, Zhou L, et al. Significance of serum microRNA-21 in diagnosis of hepatocellular carcinoma (HCC): clinical analyses of patients and an HCC rat model. Int J Clin Exp Pathol. 2015;8(2):1466–1478.
87. Zhou G, Zeng Y, Luo Y, et al. Urine miR-93-5p is a promising biomarker for early detection of HBV-related hepatocellular carcinoma. Eur J Surg Oncol. 2022;48(1):95–102. doi:10.1016/j.ejso.2021.06.015
88. Rui T, Zhang X, Guo J, et al. Serum-Exosome-Derived miRNAs Serve as Promising Biomarkers for HCC Diagnosis. Cancers. 2022;15(1):205. doi:10.3390/cancers15010205
89. Wang S, Yang Y, Sun L, et al. Exosomal MicroRNAs as Liquid Biopsy Biomarkers in Hepatocellular Carcinoma. Onco Targets Ther. 2020;13:2021–2030. doi:10.2147/OTT.S232453
90. Li F, Wang F, Zhu C, et al. miR-221 suppression through nanoparticle-based miRNA delivery system for hepatocellular carcinoma therapy and its diagnosis as a potential biomarker. Int J Nanomed. 2018;13:2295–2307. doi:10.2147/IJN.S157805
91. Mohamed AA, Omar AAA, El-Awady RR, et al. MiR-155 and MiR-665 role as potential non-invasive biomarkers for hepatocellular carcinoma in Egyptian patients with chronic hepatitis c virus infection. J Transl Int Med. 2020;8(1):32–40. doi:10.2478/jtim-2020-0006
92. Ghosh S, Bhowmik S, Majumdar S, et al. The exosome encapsulated microRNAs as circulating diagnostic marker for hepatocellular carcinoma with low alpha-fetoprotein. Int J Cancer. 2020;147(10):2934–2947. doi:10.1002/ijc.33111
93. El-Maraghy SA, Adel O, Zayed N, et al. Circulatory miRNA-484, 524, 615 and 628 expression profiling in HCV mediated HCC among Egyptian patients; implications for diagnosis and staging of hepatic cirrhosis and fibrosis. J Adv Res. 2020;22:57–66. doi:10.1016/j.jare.2019.12.002
94. Cui Y, Xu HF, Liu MY, et al. Mechanism of exosomal microRNA-224 in development of hepatocellular carcinoma and its diagnostic and prognostic value. World J Gastroenterol. 2019;25(15):1890–1898. doi:10.3748/wjg.v25.i15.1890
95. Han J, Li J, Qian Y, et al. Identification of plasma miR-148a as a noninvasive biomarker for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2019;43(5):585–593. doi:10.1016/j.clinre.2018.12.008
96. Weis A, Marquart L, Calvopina DA, et al. Serum MicroRNAs as biomarkers in hepatitis c: preliminary evidence of a MicroRNA panel for the diagnosis of hepatocellular carcinoma. Int J Mol Sci. 2019;20(4):864. doi:10.3390/ijms20040864
97. Oura K, Fujita K, Morishita A, et al. Serum microRNA-125a-5p as a potential biomarker of HCV-associated hepatocellular carcinoma. Oncol Lett. 2019;18(1):882–890. doi:10.3892/ol.2019.10385
98. Chen S, Chen H, Gao S, et al. Differential expression of plasma microRNA-125b in hepatitis b virus-related liver diseases and diagnostic potential for hepatitis b virus-induced hepatocellular carcinoma. Hepatol Res. 2017;47(4):312–320. doi:10.1111/hepr.12739
99. Chen Y, Chen J, Liu Y, et al. Plasma miR-15b-5p, miR-338-5p, and miR-764 as biomarkers for hepatocellular carcinoma. Med Sci Monit. 2015;21:1864–1871. doi:10.12659/MSM.893082
100. Chen S, Fu Z, Wen S, et al. Expression and Diagnostic Value of miR-497 and miR-1246 in Hepatocellular Carcinoma. Front Genet. 2021;12:666306. doi:10.3389/fgene.2021.666306
101. Youssef SS, Elfiky A, Nabeel MM, et al. Assessment of circulating levels of microRNA-326, microRNA-424, and microRNA-511 as biomarkers for hepatocellular carcinoma in Egyptians. World J Hepatol. 2022;14(8):1562–1575. doi:10.4254/wjh.v14.i8.1562
102. Lee YR, Kim G, Tak WY, et al. Circulating exosomal noncoding RNAs as prognostic biomarkers in human hepatocellular carcinoma. Int J Cancer. 2019;144(6):1444–1452. doi:10.1002/ijc.31931
103. Zhang W, Jiang B, Zhu H, et al. miR-33b in human cancer: mechanistic and clinical perspectives. Biomed Pharmacother. 2023;161:114432. doi:10.1016/j.biopha.2023.114432
104. Shen J, Liang C, Su X, et al. Dysfunction and ceRNA network of the tumor suppressor miR-637 in cancer development and prognosis. Biomark Res. 2022;10(1):72. doi:10.1186/s40364-022-00419-8
105. Shehab-Eldeen S, Metwaly MF, Saber SM, et al. MicroRNA-29a and MicroRNA-124 as novel biomarkers for hepatocellular carcinoma. Dig Liver Dis. 2023;55(2):283–290. doi:10.1016/j.dld.2022.04.015
106. Pelizzaro F, Cardin R, Sartori A, et al. Circulating MicroRNA-21 and MicroRNA-122 as Prognostic Biomarkers in Hepatocellular Carcinoma Patients Treated with Transarterial Chemoembolization. Biomedicines. 2021;9(8):890. doi:10.3390/biomedicines9080890
107. Jia YZ, Liu J, Wang GQ, et al. miR-484: a potential biomarker in health and disease. Front Oncol. 2022;12:830420. doi:10.3389/fonc.2022.830420
108. Wang H, Lin X, Liu E, et al. MicroRNA-33b regulates hepatocellular carcinoma cell proliferation, apoptosis, and mobility via targeting Fli-1-mediated Notch1 pathway. J Cell Physiol. 2020;235(10):7635–7644. doi:10.1002/jcp.29673
109. Yang TB, Yi F, Liu WF, et al. Identification of hsa-circ_0039053 as an up-regulated and oncogenic circRNA in hepatocellular carcinoma via the miR-637-mediated USP21 activation. J Cancer. 2020;11(23):6950–6959. doi:10.7150/jca.48998
110. Pratama MY, Visintin A, Crocè LS, et al. Circulatory miRNA as a biomarker for therapy response and disease-free survival in hepatocellular carcinoma. Cancers. 2020;12(10):2810. doi:10.3390/cancers12102810
111. Cho HJ, Kim JK, Nam JS, et al. High circulating microRNA-122 expression is a poor prognostic marker in patients with hepatitis b virus-related hepatocellular carcinoma who undergo radiofrequency ablation. Clin Biochem. 2015;48(16–17):1073–1078. doi:10.1016/j.clinbiochem.2015.06.019
112. Kim SS, Nam JS, Cho HJ, et al. Plasma micoRNA-122 as a predictive marker for treatment response following transarterial chemoembolization in patients with hepatocellular carcinoma. J Gastroenterol Hepatol. 2017;32(1):199–207. doi:10.1111/jgh.13448
113. Li T, Yin J, Yuan L, et al. Downregulation of microRNA-139 is associated with hepatocellular carcinoma risk and short-term survival. Oncol Rep. 2014;31(4):1699–1706. doi:10.3892/or.2014.3032
114. Zhao R, Fu J, Zhu L, et al. Designing strategies of small-molecule compounds for modulating non-coding RNAs in cancer therapy. J Hematol Oncol. 2022;15(1):14. doi:10.1186/s13045-022-01230-6
115. Zhou X, Jiao L, Qian Y, et al. Repositioning azelnidipine as a dual inhibitor targeting CD47/SIRPα and TIGIT/PVR pathways for cancer immunotherapy. Biomolecules. 2021;11(5):706. doi:10.3390/biom11050706
116. Dang CV, Reddy EP, Shokat KM, et al. Drugging the ‘undruggable’ cancer targets. Nat Rev Cancer. 2017;17(8):502–508. doi:10.1038/nrc.2017.36
117. Costales MG, Childs-Disney JL, Haniff HS, et al. How we think about targeting RNA with small molecules. J Med Chem. 2020;63(17):8880–8900. doi:10.1021/acs.jmedchem.9b01927
118. Mollaei H, Safaralizadeh R, Rostami Z. MicroRNA replacement therapy in cancer. Cell Physiol. 2019;234(8):12369–12384. doi:10.1002/jcp.28058
119. Robb T, Reid G, Blenkiron C. Exploiting microRNAs As Cancer Therapeutics. Target Oncol. 2017;12(2):163–178. doi:10.1007/s11523-017-0476-7
120. Hong DS, Kang YK, Borad M, et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br J Cancer. 2020;122(11):1630–1637. doi:10.1038/s41416-020-0802-1
121. Thakral S, Ghoshal K. miR-122 is a unique molecule with great potential in diagnosis, prognosis of liver disease, and therapy both as miRNA mimic and antimir. Curr Gene Ther. 2015;15(2):142–150. doi:10.2174/1566523214666141224095610
122. Shi Z, Zhang J, Qian X, et al. AC1MMYR2, an inhibitor of dicer-mediated biogenesis of oncomir miR-21, reverses epithelial-mesenchymal transition and suppresses tumor growth and progression. Cancer Res. 2013;73(17):5519–5531. doi:10.1158/0008-5472.CAN-13-0280
123. Hassan M, Elzallat M, Aboushousha T, et al. MicroRNA-122 mimic /microRNA-221 inhibitor combination as a novel therapeutic tool against hepatocellular carcinoma. Non-Coding RNA Research. 2022;8(1):126–134. doi:10.1016/j.ncrna.2022.11.005