Monitoring the Antibacterial Activity of the Green Synthesized ZnO Nanoparticles on the Negative and Positive Gram Bacteria Mimicking Oral Environment by Using a Quartz Tuning Fork (QTF) Micromechanical Sensor

Introduction
Metal oxide nanoparticles have potential applications in sensors, medical delivery, medical imaging, and antibacterial and anticancer applications.1–10 The antibacterial properties of ZnO nanoparticles have been used in various dental applications such as ortho wires, resto/endo materials, and oral bite guard materials.11 Green-synthesized ZnO nanoparticles (NPs) function as antibacterial materials and exhibit high growth inhibition.12 ZnO NPs have been reported to reduce the growth of several harmful bacteria.13 The size and shape of ZnO nanoparticles have a major influence on their antibacterial activity. It has been demonstrated that the antibacterial activity of ZnO is inversely proportional to its nanoparticle size.14 The antibacterial activity of ZnO nanoparticles has been attributed to a variety of mechanisms such as the creation of reactive oxygen species (ROS),15–18 direct contact between ZnO nanoparticles and cell walls,19–21 and the release of Zn2+.15,22 ROS is produced upon exposure to light. In contrast, alternative investigations have revealed that ZnO exhibits antibacterial activity, even in the dark.23–26 The release of Zn2+ in a solution containing bacteria and ZnO nanoparticles was hypothesized to be one of the ZnO antibacterial mechanisms through the inhibition of active transport, disruption of enzyme systems, and effects on amino acid metabolism.15,22,27 Nevertheless, the contribution of Zn2+ to the antibacterial effect of ZnO nanoparticles is low, owing to the small amount of soluble Zn released from ZnO in water.28,29 Conversely, it has been claimed that ROS are formed in the dark by capturing electrons that react with dissolved oxygen via oxygen vacancies associated with defects to produce ROS.24 In the dark, ROS are formed as hydrogen peroxide, superoxide radicals, or both without the production of hydroxyl radicals.24,25 The ROS produced act as antibacterial agents.24–26,30,31 In contrast, the direct contact of ZnO nanoparticles with bacterial walls results in breakdown of the bacterial cell structure.32 There are still unanswered questions regarding the chemical mechanism by which nanoparticles cross the outer membrane and cell wall before causing damage to bacteria. It has been discovered that gram-negative bacteria exhibit greater vulnerability to the nanoparticles than gram-positive bacteria.33–35 This variation in susceptibility was assumed to be caused by variations in cell wall composition. One theory argues that gram-negative bacteria are more prone to cell wall breakdown when they come into contact with nanoparticles because of their thin peptidoglycan covering. Gram-negative bacteria also have an outer membrane consisting of lipopolysaccharides, which carry a negative charge. The presence of positively charged nanoparticles in the outer membrane can result in their accumulation, causing damage to bacterial cells.4,35 Furthermore, the size and structure of nanoparticles have a significant impact on the antibacterial activity of ZnO.32,36 Smaller ZnO nanoparticles have been demonstrated to be more bactericidal than larger ones, possibly because smaller particles are more easily absorbed and transfected across the cell membrane. Furthermore, the larger surface-area-to-volume ratio of smaller particles may result in the formation of more reactive oxygen species.37 ZnO nanoparticles are promising antimicrobial coating materials for biomedical applications because of their antibacterial activity under dark conditions, biocompatibility, and non-toxicity to humans.38–41 ZnO nanoparticles can be used as antibacterial coatings for oral bite guards, as illustrated in Figure 1.
|
Figure 1 Schematic diagram for a coated oral bite guard. |
Owing to the growing interest in the properties of new antimicrobial products, it is essential to develop cost-effective, real-time, sensitive, and quantitative antimicrobial testing methods. There are several antimicrobial testing methods, such as disc diffusion, thin layer Chromatography, Dilution, adenosine triphosphate (ATP) bioluminescence assays, and flow cytometry.40 Diffusion techniques such as the agar disk diffusion method are qualitative tests. However, they have notable limitations, including issues with precision, reproducibility, and interpretation, as well as difficulties in quantifying the amount of diffused antimicrobial agent in agar medium. Additionally, thin-layer chromatography (TLC) bioautography methods are time-consuming and labor-intensive. Dilution methods are used to determine the minimum inhibitory concentration (MIC) of an antimicrobial agent. Similarly, ATP bioluminescence assays and flow cytometry are labor-intensive techniques. An MEMS-based microchannel cantilever was utilized to monitor nanomechanical fluctuations and bacterial bacteriophage interactions.42 Quartz tuning fork (QTF) micromechanical sensors are a potential technology for biosensing owing to their low cost, high sensitivity, simplicity, and rapid detection. The QTF-based micromechanical sensor is a sensitive and precise technique that operates on the principle of vibrating two prongs of a quartz crystal; it vibrates at its resonant frequency when a voltage is applied to the electrodes on the quartz. The principle of the QTF-based sensor depends on the shifting of its mechanical resonance frequency, which is used as an indication of the physical, chemical, or biological interactions with high precession and sensitivity. The QTF surface can be functionalized by host molecules that can selectively bind to target molecules, resulting in mass loading and shifting the QTF resonance frequency to lower values.43–45 The QTF resonance frequency increases as the number of target molecules attracted to the probe increases. In addition, the resonance frequency and quality factor of a vibrating QTF can also be changed if it is immersed in different media with different physical properties.46 QTF can be used to monitor and diagnose diseases caused by cytomegalovirus (CMV) and germ-like bacteria.45,47 This work introduced a new antibacterial test method based on a QTF-system biosensor to monitor the antibacterial activity of nanoparticles under controlled conditions and at low volumes on the microliter scale. The QTF is coast-effective, highly sensitive, and works at microscale liquid volumes. Therefore, it can be used to detect low concentrations of bacteria and nanoparticle antibacterial activity in both liquid and wet media. This study aimed to investigate the ability of a QTF-based biosensor to detect the antibacterial activity of green-synthesized ZnO nanoparticles using Rosmarinus officinalis L. extract in the oral environment of Gram-positive and Gram-negative bacteria, which can be used as antibacterial oral bite guards. The green synthesis method of metal oxide nanoparticles is a cost-effective, eco-friendly solvent, and yields productivity on a large scale.48 In addition, green-synthesized nanoparticles are more biocompatible and soluble in water.49 The using of Rosmarinus officinalis extract was used because it contains many phenolic compounds that reduce and stabilize nanoparticles.
Experimental Procedure
Materials
Green synthesized ZnO nanoparticles using Rosmarinus officinalis L. extract and healthy wild Rosmarinus officinalis L. (KSU Herbarium No: 9012) leaves were collected from the Abha region in southern Saudi Arabia. Zinc nitrate hexahydrate and sodium hydroxide were obtained from BHD® (Avantor, Inc., Radnor, PV, U.S). All chemicals were used without further purification. Bacterial growth media (Nutrient Broth from Scharluau microbiology), Escherichia coli bacteria code (ATCC25922)102 CFU/mL, Staphylococcus aureus bacteria code (ATCC29213)102 CFU/mL, and QTF sensors.
Green Synthesis Nanoparticles Preparation for Antibacterial Test
Rosmarinus Officinalis Extract Preparation
Leaf extract was prepared following the method described by Saad Algarni et al (2024).50 Fresh leaves were washed, shadow-dried for 2–3 weeks, and then ground. The solution extract was prepared by dissolving 10 g of leaf powder in 100 mL deionized water and heating at 60 °C for 15 min, then filtering and storing at 4 °C.
Synthesis of ZnO NPs Using Rosmarinus Leaf Extract
The green-synthesized ZnO nanoparticles (NPs) were prepared according to the method published by T. Saad Algarniet al (2024).50 Zinc nitrate hexahydrate (5 g) was dissolved in 30 mL of aqueous leaf extract and 18 mL of 2M NaOH was added to achieve a pH of 5.2. The mixture was heated to 80 °C and stirred for 3 h. Subsequently, the precipitate material was separated, washed multiple times with a distilled water-ethanol (3:1) solution, and then dried at 80 °C overnight. Finally, the product material was calcined at 500 °C for 3 h to obtain the ZnO NPs. Figure 2 shows the microstructural Scanning electron microscopy (SEM) image and Energy dispersive x-ray spectroscopy (EDS) of green-synthesized ZnO, the particle size in the range of 53–67 nm.
 |
Figure 2 (a) SEM image (b) EDS spectrum of the green synthesized ZnO nanoparticle. |
Bacteria Growth and Antibacterial Test
The antibacterial activity of the green-synthesized ZnO NPs against gram-positive and gram-negative bacteria was assessed. S. aureus (ATCC 25923) and E. coli (ATCC 25922) were used as gram-positive and gram-negative bacteria, respectively. The ZnO NPs were suspended in sterilized deionized (DI) water using ultrasonic vibrations at a frequency of 45 kHz for 1 h and then added to the growth media (Nutrient Broth, PH of 7.4) at different concentrations (1, 0.5, 0.25, and 0.125 mm). Bacteria with a starting concentration of 102 CFU/mL were grown individually in growth media with different concentrations of ZnO nanoparticles (1, 0.5, 0.25, 0.125, and 0 mm). All the bacterial samples were incubated in the dark at 37 °C with continuous shaking for 8 h.
QTF Antibacterial Measurements Test
The sensing part of the QTF sensor was treated with UV-ozone for 15 min, followed by immersion in a Piranha H2SO4:H2O2 (3:1) solution for 5 min, rinsing with DI water three times, twice with absolute ethanol, and drying using high-purity dry nitrogen. To investigate the antibacterial activity of the ZnO nanoparticles, the QTF-based biosensor was immersed in 100 µL of bacteria grown in media containing a certain concentration of ZnO nanoparticles. The sensor response was measured as the resonance frequency shift and the quality factor of the QTF sensor. The resonance frequency was measured after immersing the QTF sensor in a bacterial sample, as illustrated in Figure 3. Measurements were performed after 5 min for each sample. The resonance frequency of the QTF samples was measured using a Quester-10 QTF measurement system from FOURIEN Company, Canada, which has been described in more detail in the literature.46
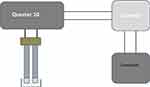 |
Figure 3 Schematic diagram for the QTF based antibacterial tests system. |
Results and Discussion
Results
The antibacterial activity of the green-synthesized ZnO nanoparticles was evaluated using the resonance frequency of the QTF-based biosensor. The variation in the resonance frequency of the QTF immersed in the bacterial solution with different contents of green-synthesized ZnO nanoparticles was measured as an indicator of the antibacterial activity of the nanoparticles. The resonance frequency and quality factor variations reflect changes in the bacterial concentration. Figure 4a shows the resonance frequency of the QTF immersed in E. coli grown in growth media containing different concentrations of ZnO nanoparticles (0, 0.125, 0.25, 0.5, and 1 mm). As the ZnO nanoparticles content in the bacterial solution increased, the resonance frequency of the QTF also increased. Conversely, a decrease in the ZnO nanoparticles content in the bacterial solution resulted in a decrease in the resonance frequency. The resonance frequency of the QTF sensor immersed in E. coli bacteria solution without ZnO nanoparticles incubated for 8 h decreased by 199.64±2.64 hz compared to that immersed in the growth media. On the other hand, the resonance frequency of the QTF sensor immersed in E. coli solution media with 1 mm ZnO nanoparticles incubated for 8 h decreased by 30.57±4.01 hz. The resonance frequency shift (Δf) was determined by subtracting the resonance frequency of the sensor immersed in the bacterial solution (fB) from the resonance frequency of the QTF immersed in the growth medium (fM) according to Equation (1):
 |
Figure 4 QTF (a) resonance frequency, (b) frequency shift after immersion in E. Coli bacteria growth in different ZnO nanoparticles content. |

Clearly, the resonance frequency shift increased as the concentration of ZnO nanoparticles decreased, as illustrated in Figure 4b. Furthermore, the quality factor increased as the concentration of ZnO nanoparticles increased, as shown in Figure 5a and b. The quality factor of the QTF sensor decreased as the concentration of the ZnO nanoparticles decreased.
 |
Figure 5 The QTF microsensor (a) quality factor, (b) quality factor shift after immersion in E. Coli bacteria with different ZnO nanoparticles content. |
The maximum quality factor was observed when the sensor was immersed in growth media without bacteria, while the minimum value for the sensor was observed when the sensor was immersed in bacteria incubated in media without ZnO nanoparticles. As the concentration of the ZnO nanoparticles increased, the quality factor increased.
On the other hand, to assess the antibacterial activity of ZnO nanoparticles on the positive gram bacteria by using the QTF biosensor, the resonance frequency and quality factor of the QTF immersed in the in S. aureus grown in media with different concentrations of the green synthesized ZnO nanoparticles was measured as a sensor response. The resonance frequency of the QTF sensor immersed in S. aureus with 1 mm ZnO nanoparticles was lower than that of the sensor immersed in the growth media by 5.69±3.81 hz. Moreover, as shown in Figure 6, the resonance frequency shift decreased as the ZnO nanoparticle content increased. Figure 7 illustrates the quality factor and quality factor shift of the QTF sensor incubated in S. aureus with different ZnO nanoparticle concentrations. The quality factor of the sensor did not change when the sensor was incubated with S. aureus and 1 mm ZnO nanoparticles.
 |
Figure 6 QTF (a) resonance frequency curves (b) frequency shift after immersion in S. aureus bacteria growth different ZnO nanoparticles content. |
 |
Figure 7 (a) quality factor (b) quality factor variation of the QTF microsensor immersed in S. aureus with different ZnO nanoparticle contents. |
In comparison, the resonance frequency and quality factor of the sensor shifted more for the QTF immersed in E. coli bacteria than S. aureus at the same concentration of ZnO nanoparticles.
Discussion
The lower reduction in resonance frequency and quality factor values for the bacteria grown in higher ZnO nanoparticle concentrations indicates a decrease in the bacterial concentration in the solution. The resonance frequency and quality factor shift can be used as a response to bacterial concentration variations in growth media. The bacterial concentration decreased as the green-synthesized ZnO nanoparticle content increased due to the antibacterial effect, which killed the bacteria and inhibited their growth. Conversely, the resonance frequency decreased as the concentration of ZnO nanoparticles increased with an increase in the number of bacteria attached to the sensor surface. Bacteria have a high ability to attach to the silicon oxide surface. Therefore, as the concentration of bacteria increases, the resonance frequency decreases because of the increase in mass loading resulting from the attachment of bacteria to silicon oxide on the QTF surface.45,51 The attachment of bacteria to the surface is related to several mechanisms, such as adhesion pili, Van der Waals forces, and hydrogen bonds, and zeta potential also plays a role in cell adhesion.51–53 The decrease in the quality factor at lower ZnO concentrations and higher bacterial concentrations can be attributed to the increased viscosity of the solution caused by the growing bacterial population. This increased viscosity leads to a greater damping effect on the QTF. Additionally, the change in quality factor can serve as a useful indicator of bacterial concentration, as it reflects the interaction between the sensor and the bacteria in the growth solution. The observed differences in resonance frequency and quality factor shifts between S. aureus and E. coli can be attributed to the different antibacterial effects of Rosmarinus officinalis-synthesized ZnO nanoparticles on gram-positive and gram-negative bacteria. The lower resonance frequency shift in S. aureus suggests that the Rosmarinus officinalis synthesized ZnO nanoparticles have a more significant impact on S. aureus gram-positive bacteria than on E. coli gram-negative bacteria due to differences in cell wall structure or nanoparticle interactions. The green Rosmarinus officinalis synthesized ZnO nanoparticles exhibited antibacterial effects against both Sauries and E. coli. However, S. aureus exhibited a slightly higher susceptibility to the ZnO nanoparticles used in this study. The lower effect of ZnO nanoparticles on gram-negative bacteria is because they have a complex cell wall that consists of an outer membrane with lipopolysaccharides, a thin peptidoglycan layer, and inner lipid components; however, gram-positive bacteria possess simpler cell walls composed mainly of thick, compact peptidoglycan layers.54 Table 1 shows some literature reports on the antibacterial effect of green-synthesized ZnO particles.
 |
Table 1 A Comparison Between Studies Using the Antibacterial Effect of Green-Synthesized ZnO Particles |
It has been reported that ZnO nanoparticles act as antibacterial materials to inhibit bacterial growth through the attachment of ZnO nanoparticles to the bacterial surface, resulting in an increase in Zn2+ in the bacterial cytoplasm.23,58 In addition, it has been reported in the literature that ROS are produced in the dark; ROS such as hydrogen peroxide and superoxide radicals are generated, while hydroxyl radicals are not produced, which act as antibacterial agents.24,25 The smaller particles can penetrate into the cell membrane and produce ROS inside the cell resulting in more effect, as well as the penetrated nanoparticles causes mechanical damage to the cell membrane resulting in the leaking of bacterial cell cytoplasm.59,60 In addition, the QTF sensor exhibited a slightly greater decrease in both resonance frequency and quality factor when immersed in E. coli without ZnO nanoparticles, indicating that E. coli may exert a stronger mechanical effect owing to its larger cell size compared to S. aureus. These findings suggest that the mechanical properties of the bacterial cells, combined with the antibacterial activity of ZnO nanoparticles, influence the response of the sensor.
Conclusion
The QTF system successfully detected the effect of the green synthesized ZnO nanoparticles on both Gram-negative (E. coli) and Gram-positive (S. aureus) bacteria in an oral-mimicking environment through the changes in resonance frequency and quality factor as a function of ZnO concentration. Therefore, the QTF micromechanical biosensor can work as a low coast, real-time, label-free, and quantitative a sensing platform to assess the antibacterial activity. The green-synthesized ZnO nanoparticle exhibited good antibacterial activity against both E. coli and S. aureus. As the concentration of ZnO nanoparticles in the growth solution increased, the resonance frequency and quality factor increased, indicating enhanced antibacterial activity and reduced bacterial presence. Notably, the QTF system revealed a stronger antibacterial effect of the green synthesized by Rosmarinus officinalis ZnO nanoparticles on S. aureus than E. coli, demonstrating its potential for comparative microbial studies. The work exhibited the ability of the QTF micromechanical sensor to convert nanoscale biological interactions into measurable mechanical signals in a short time, eliminating the need for dyes, biomarkers, and lengthy incubation steps required in the conventional methods. In conclusion, the QTF sensor can work as a powerful alternative technology for label-free, real-time, and miniaturized antimicrobial effects suitable for both research and potential clinical applications.
Acknowledgments
The authors extend their appreciation to the deputyship for research and innovation, Ministry of Education in Saudi Arabia, for funding this research work through project number (IFP-2022-11).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saddik MS, Elsayed MMA, Abdelkader MSA, et al. Novel green biosynthesis of 5-fluorouracil chromium nanoparticles using harpullia pendula extract for treatment of colorectal cancer. Pharmaceutics. 2021;13:226. doi:10.3390/pharmaceutics13020226
2. Wang Y, Wang DN, Yang M, Hong W, Lu P. Refractive index sensor based on a microhole in single-mode fiber created by the use of femtosecond laser micromachining. Optics Letters. 2009;34:3328. doi:10.1364/ol.34.003328
3. Hajipour MJ, Fromm KM, Ashkarran AA, et al. Antibacterial properties of nanoparticles. Trends in Biotechnology. 2012;30:499–511. doi:10.1016/j.tibtech.2012.06.004
4. Slavin YN, Asnis J, Hńfeli UO, Bach H. Metal nanoparticles: understanding the mechanisms behind antibacterial activity. Journal of Nanobiotechnology. 2017;15:1–20. doi:10.1186/s12951-017-0308-z
5. Bruna T, Maldonado-Bravo F, Jara P, Caro N. Silver nanoparticles and their antibacterial applications. International Journal of Molecular Sciences. 2021;22:7202. doi:10.3390/ijms22137202
6. Khan MI, Shah S, Faisal S, et al. Monotheca buxifolia driven synthesis of zinc oxide nano material its characterization and biomedical applications. Micromachines. 2022;13:668. doi:10.3390/mi13050668
7. Zafar S, Faisal S, Jan H, et al. Development of iron nanoparticles (FeNPs) using biomass of Enterobacter: its characterization, antimicrobial, anti-Alzheimer’s, and enzyme inhibition potential. Micromachines. 2022;13:1259. doi:10.3390/mi13081259
8. Alsaedi WH, Mohamed WS, Qasem HA, et al. Fabrication of CuO/PdO nanocomposites for biomedical applications. Inorganic Chemistry Communications. 2024;170:113166. doi:10.1016/j.inoche.2024.113166
9. Khalil KD, Bashal AH, Habeeb T, Abu‐Dief AM. Synergistic antibacterial and anticancer activity in gadolinium–chitosan nanocomposite films: a novel approach for biomedical applications. Applied Organometallic Chemistry. 2024;38:e7531. doi:10.1002/aoc.7531
10. Abu-Dief AM, Alsehli M, Awaad A. The bioreaction and immune responses of PEG-coated silica NPs and the role of the surface density coating after oral administration into mice. Applied Nanoscience. 2023;13:5563–5578. doi:10.1007/s13204-023-02770-0
11. Pushpalatha C, Suresh J, Gayathri VS, et al. Zinc oxide nanoparticles: a review on its applications in dentistry. Frontiers in Bioengineering and Biotechnology. 2022;10:917990. doi:10.3389/fbioe.2022.917990
12. Gunalan S, Sivaraj R, Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens, progress in natural science. Materials International. 2012;22:693–700.
13. Jones N, Ray B, Ranjit KT, Manna AC. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiology Letters. 2008;279:71–76. doi:10.1111/j.1574-6968.2007.01012.x
14. Raghupathi KR, Koodali RT, Manna AC. Size-dependent bacterial growth inhibition and mechanism of antibacterial activity of zinc oxide nanoparticles. Langmuir. 2011;27:4020–4028. doi:10.1021/la104825u
15. Li M, Zhu L, Lin D. Toxicity of ZnO nanoparticles to Escherichia coli: mechanism and the influence of medium components. Environmental Science & Technology. 2011;45:1977–1983. doi:10.1021/es102624t
16. Kasemets K, Ivask A, Dubourguier H-C, Kahru A. Toxicity of nanoparticles of ZnO, CuO and TiO2 to yeast Saccharomyces cerevisiae. Toxicology in Vitro. 2009;23:1116–1122. doi:10.1016/j.tiv.2009.05.015
17. Sirelkhatim A, Mahmud S, Seeni A, et al. Review on zinc oxide nanoparticles: antibacterial activity and toxicity mechanism. Nano-Micro Letters. 2015;7:219–242. doi:10.1007/s40820-015-0040-x
18. Perelshtein I, Applerot G, Perkas N, et al. Antibacterial properties of an in situ generated and simultaneously deposited nanocrystalline ZnO on fabrics. ACS Applied Materials & Interfaces. 2009;1:361–366. doi:10.1021/am8000743
19. Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fiévet F. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Letters. 2006;6:866–870. doi:10.1021/nl052326h
20. Zhang L, Jiang Y, Ding Y, Povey M, York D. Investigation into the antibacterial behaviour of suspensions of ZnO nanoparticles (ZnO nanofluids. Journal of Nanoparticle Research. 2007;9:479–489. doi:10.1007/s11051-006-9150-1
21. Adams LK, Lyon DY, Alvarez PJJ. Comparative eco-toxicity of nanoscale TiO2, SiO2, and ZnO water suspensions. Water Research. 2006;40:3527–3532. doi:10.1016/j.watres.2006.08.004
22. Premanathan M, Karthikeyan K, Jeyasubramanian K, Manivannan G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine: Nanotechnology, Biology and Medicine. 2011;7:184–192. doi:10.1016/j.nano.2010.10.001
23. Joe A, Park S-H, Shim K-D, et al. Antibacterial mechanism of ZnO nanoparticles under dark conditions. Journal of Industrial and Engineering Chemistry. 2017;45:430–439. doi:10.1016/j.jiec.2016.10.013
24. Haddada EB, Karkouch I, Hamraoui K, et al. Highly efficient antibacterial activity in the dark and under UV illumination of ZnO nanoplates dispersed in water. Emergent Materials. 2023;6:1503–1517. doi:10.1007/s42247-023-00546-4
25. Biswas A, Kar U, Jana NR. Cytotoxicity of ZnO nanoparticles under dark conditions via oxygen vacancy dependent reactive oxygen species generation. Physical Chemistry Chemical Physics. 2022;24:13965–13975. doi:10.1039/D2CP00301E
26. Zhou Y, Guo Y, Li J, et al. Excellent antibacterial activities in the dark of ZnO nanoflakes with oxygen vacancies on exposed {21 [combining macron] 1 [combining macron] 0} facets. Journal of Materials Chemistry A. 2020;8:11511–11514. doi:10.1039/C9TA14044A
27. Sevinç BA, Hanley L. Antibacterial activity of dental composites containing zinc oxide nanoparticles. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2010;94:22–31. doi:10.1002/jbm.b.31620
28. Sawai J. Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. Journal of Microbiological Methods. 2003;54:177–182. doi:10.1016/S0167-7012(03)00037-X
29. Jiang W, Mashayekhi H, Xing B. Bacterial toxicity comparison between nano-and micro-scaled oxide particles. Environmental Pollution. 2009;157:1619–1625. doi:10.1016/j.envpol.2008.12.025
30. Prasanna VL, Vijayaraghavan R. Insight into the mechanism of antibacterial activity of ZnO: surface defects mediated reactive oxygen species even in the dark. Langmuir. 2015;31:9155–9162. doi:10.1021/acs.langmuir.5b02266
31. Gupta J, Bahadur D. Defect-mediated reactive oxygen species generation in Mg-substituted ZnO nanoparticles: efficient nanomaterials for bacterial inhibition and cancer therapy. ACS Omega. 2018;3:2956–2965. doi:10.1021/acsomega.7b01953
32. Jadhav S, Gaikwad S, Nimse M, Rajbhoj A. Copper oxide nanoparticles: synthesis, characterization and their antibacterial activity. Journal of Cluster Science. 2011;22:121–129. doi:10.1007/s10876-011-0349-7
33. Cavassin ED, de Figueiredo LFP, Otoch JP, et al. Comparison of methods to detect the in vitro activity of silver nanoparticles (AgNP) against multidrug resistant bacteria. Journal of Nanobiotechnology. 2015;13:1–16. doi:10.1186/s12951-015-0120-6
34. Mukha IP, Eremenko AM, Smirnova NP, et al. Antimicrobial activity of stable silver nanoparticles of a certain size. Applied Biochemistry and Microbiology. 2013;49:199–206. doi:10.1134/S0003683813020117
35. Surwade P, Luxton T, Clar J, Xin F, Shah V. Impact of the changes in bacterial outer membrane structure on the anti-bacterial activity of zinc oxide nanoparticles. Journal of Nanoparticle Research. 2020;22:1–8. doi:10.1007/s11051-020-4767-z
36. Shahid S, Khan SA, Ahmad W, Knawal S. Size-dependent bacterial growth inhibition and antibacterial activity of ag-doped ZnO nanoparticles under different atmospheric conditions. Indian Journal of Pharmaceutical Sciences. 2018;80. doi:10.4172/pharmaceutical-sciences.1000342
37. Rutherford D, Jíra J, Kolářová K, et al. Growth inhibition of gram-positive and gram-negative bacteria by zinc oxide hedgehog particles. International Journal of Nanomedicine. 2021;Volume 16:3541–3554. doi:10.2147/IJN.S300428
38. Ramesh P, Saravanan K, Manogar P, Johnson J, Vinoth E, Mayakannan M. Green synthesis and characterization of biocompatible zinc oxide nanoparticles and evaluation of its antibacterial potential. Sensing and Bio-Sensing Research. 2021;31:100399. doi:10.1016/j.sbsr.2021.100399
39. Quintão CCR, Saraiva NZ, Oliveira CS, et al. Antioxidant effects and compatibility of zinc oxide nanoparticles during in vitro maturation of bovine oocytes and subsequent embryo development. Theriogenology. 2024;230:1–7. doi:10.1016/j.theriogenology.2024.08.033
40. Balouiri M, Sadiki M, Ibnsouda SK. Methods for in vitro evaluating antimicrobial activity: a review. Journal of Pharmaceutical Analysis. 2016;6:71–79. doi:10.1016/j.jpha.2015.11.005
41. Puspasari V, Ridhova A, Hermawan A, Amal MI, Khan MM. ZnO-based antimicrobial coatings for biomedical applications. Bioprocess and Biosystems Engineering. 2022;45:1421–1445. doi:10.1007/s00449-022-02733-9
42. Alzahrani KE, Alodhayb A, Algwati M, et al. Nanomechanical detection of bacteria–bacteriophage interactions using microchannel microcantilevers. Journal of the Electrochemical Society. 2021;168:87509. doi:10.1149/1945-7111/ac1dcf
43. Al-Gawati M, Ain QT, Alzahrani KE, et al. Label free detection of programmed death ligand 1 protein biomarker by quartz tuning fork-based biosensor. Journal of the Electrochemical Society. 2023;170:127511. doi:10.1149/1945-7111/ad11ad
44. Rahman S, Al-Gawati MA, Alfaifi FS, et al. The effect of counterions on the detection of cu2+ ions in aqueous solutions using Quartz Tuning Fork (QTF) sensors modified with l-cysteine self-assembled monolayers. Experimental and Quantum Chemical DFT Study, Chemosensors. 2023;11:88.
45. Alshammari A, Abdulmawla ST, Alsaigh R, et al. Toward the real-time and rapid quantification of bacterial cells utilizing a quartz tuning fork sensor. Micromachines. 2023;14:1114. doi:10.3390/mi14061114
46. Alodhayb A. Quartz tuning fork, a low-cost orthogonal measurement tool for the characterization of low-volume liquid reagents. Measurement. 2020;152:107313. doi:10.1016/j.measurement.2019.107313
47. Assaifan AK, Al-Gawati MA, Alzahrani KE, et al. Quartz tuning fork-based biosensor for the direct detection of human cytomegalovirus. Journal of King Saud University-Science. 2023;102703. doi:10.1016/j.jksus.2023.102703
48. Ocsoy I, Tasdemir D, Mazicioglu S, Tan W. Nanotechnology in plants. Plant Genetics and Molecular Biology. 2018;263–275.
49. Demirbas A, Kislakci E, Karaagac Z, Onal I, Ildiz N, Ocsoy I. Preparation of biocompatible and stable iron oxide nanoparticles using anthocyanin integrated hydrothermal method and their antimicrobial and antioxidant properties. Materials Research Express. 2019;6:125011. doi:10.1088/2053-1591/ab540c
50. Algarni TS, Abduh NAY, Kahtani AA, Aouissi A. Photocatalytic degradation of some dyes under solar light irradiation using ZnO nanoparticles synthesized from Rosmarinus officinalis extract. Green Chemistry Letters and Reviews. 2022;15:460–473. doi:10.1080/17518253.2022.2089059
51. Lozins R, Selga T, Ozoliņš D. Microorganism adhesion using silicon dioxide: an experimental study, Heliyon 6. Heliyon. 2020;6:e03678. doi:10.1016/j.heliyon.2020.e03678
52. Halder S, Yadav KK, Sarkar R, et al. Alteration of Zeta potential and membrane permeability in bacteria: a study with cationic agents. SpringerPlus. 2015;4:1–14. doi:10.1186/s40064-015-1476-7
53. Cross S. Underwood’s pathology. Elsevier Health Sciences. 2013.
54. El-Khawaga AM, Elsayed MA, Gobara M, et al. Green synthesized ZnO nanoparticles by Saccharomyces cerevisiae and their antibacterial activity and photocatalytic degradation. Biomass Conversion and Biorefinery. 2025;15:2673–2684. doi:10.1007/s13399-023-04827-0
55. Stan M, Popa A, Toloman D, Silipas T-D, Vodnar DC. Antibacterial and antioxidant activities of ZnO nanoparticles synthesized using extracts of Allium sativum. Rosmarinus Officinalis and Ocimum Basilicum, Acta Metallurgica Sinica (English Letters). 2016;29:228–236. doi:10.1007/s40195-016-0380-7
56. Naseer M, Aslam U, Khalid B, Chen B. Green route to synthesize zinc oxide nanoparticles using leaf extracts of cassia fistula and Melia azadarach and their antibacterial potential. Scientific Reports. 2020;10:9055. doi:10.1038/s41598-020-65949-3
57. MuthuKathija M, Badhusha MSM, Rama V. Green synthesis of zinc oxide nanoparticles using pisonia alba leaf extract and its antibacterial activity. Applied Surface Science Advances. 2023;15:100400. doi:10.1016/j.apsadv.2023.100400
58. Motelica L, Oprea O-C, Vasile B-S, et al. Antibacterial activity of solvothermal obtained ZnO nanoparticles with different morphology and photocatalytic activity against a dye mixture: methylene blue, rhodamine B and methyl Orange. International Journal of Molecular Sciences. 2023;24:5677. doi:10.3390/ijms24065677
59. Abebe B, Zereffa EA, Tadesse A, Murthy HCA. A review on enhancing the antibacterial activity of ZnO: mechanisms and microscopic investigation. Nanoscale Research Letters. 2020;15:1–19. doi:10.1186/s11671-020-03418-6
60. Motelica L, Vasile B-S, Ficai A, et al. Influence of the alcohols on the zno synthesis and its properties: the photocatalytic and antimicrobial activities. Pharmaceutics. 2022;14:2842. doi:10.3390/pharmaceutics14122842



