A Case Report of Wound Infection Caused by Mycobacterium porcinum
1Department of Clinical Laboratory, Fuwai Hospital Chinese Academy of Medical Sciences, Shenzhen, People’s Republic of China; 2Department of Clinical Research and Epidemiology, Shenzhen Institute for Cardiovascular Disease, Shenzhen, People’s Republic of China
Correspondence: Houqing Zhou, Department of Clinical Research and Epidemiology, Shenzhen Institute for Cardiovascular Disease, No. 12, Langshan Road, Nanshan District, Shenzhen, 518000, People’s Republic of China, Tel +86 821 800 28-8513, Email [email protected]
Background: Mycobacterium porcinum is a rapidly growing non-tuberculous mycobacterium that requires 3 to 5 days of cultivation for colonies to become visible to the naked eye, with most exhibiting vigorous growth during this initial week. It does not readily grow in standard bacterial culture conditions, posing challenges that often result in missed or incorrect diagnoses, and necessitating an extended course of anti-infective treatment.
Case Presentation: This case report describes a rare instance of wound infection caused by Mycobacterium porcinum in a 64-year-old female patient who underwent mitral valve bioprosthesis replacement and temporary cardiac pacemaker implantation. In the postoperative period, the patient exhibited signs of infection, including elevated leukocyte count and neutrophil percentage, along with serous discharge from the surgical wound. Mycobacterium porcinum was definitively identified using 16sRNA gene sequencing. The patient responded well to a combination therapy consisting of amikacin, azithromycin, and moxifloxacin, complemented by surgical debridement.
Conclusion: Rapidly growing mycobacteria, which require extended cultivation periods for visible colony formation, often result in underdetection. The atypical presentation of clinical symptoms can further delay the initiation of appropriate antibiotic therapy. This case underscores the critical role of prolonged cultivation time in detecting rapidly growing mycobacteria and highlights the importance of confirming genuine infections and the precision of clinical medication based on clinical symptoms and treatment outcomes.
Keywords: Mycobacterium porcinum, wound infection, postoperative complications, non-tuberculous mycobacteria, antimicrobial therapy, case report
Introduction
Mycobacterium porcinum, a rapidly growing non-tuberculous mycobacterium, was classified from the Mycobacterium fortuitum group. These mycobacteria are distinguished by their ability to form visible colonies within 3 to 5 days of incubation, with the majority exhibiting robust growth within the first week.1 They are omnipresent in various environmental niches, including water sources, soil, and carriage in animals, and can be transmitted through aerosols, dust particles, or via contact with contaminated water and soil, classifying them as opportunistic pathogens.2 The conventional bacterial culture methods frequently fail to isolate these organisms, potentially leading to misdiagnoses or oversights, and their management necessitates an extended course of anti-infective therapy. In addition to conventional antibiotic therapy, surgical debridement plays a crucial role in the treatment of rapidly growing mycobacterial wound infections.3 A literature review revealed that naturally derived multifunctional hydrogels can be applied to wound sites to modulate the inflammatory microenvironment and macrophage polarization, thereby achieving anti-inflammatory and antibacterial effects while promoting tissue repair.4 Robot-assisted debridement is an emerging therapeutic modality for cavitary and luminal infectionsThis article reports a case of wound infection caused by Mycobacterium porcinum in a diabetic patient, investigating the infection mechanisms and wound healing approaches to emphasize the critical importance of accurate diagnosis and appropriate treatment strategies in clinical practice.
Case Presentation
A 64-year-old female was admitted to the cardiovascular surgery department on July 5, 2022, presenting with symptoms of shortness of breath, palpitations, and fever following activity for over 10 days. Her admission vital signs included a temperature of 36.5°C, a heart rate of 83 beats per minute, a respiratory rate of 22 breaths per minute, and a blood pressure of 106/59 mmHg. The patient was conscious, displayed no signs of distress, had no jugular venous distension, and exhibited moist rales at the lung bases. There was no cardiac apical lift, the heart rhythm was irregular, and a grade III systolic murmur was auscultated at the left apical region; no cardiac murmurs were detected on the right side. The abdominal examination revealed a soft and non-tender abdomen, and there was no edema in the lower limbs. Chest X-ray imaging indicated bilateral pneumonia, and echocardiography confirmed the diagnoses of infective endocarditis, severe non-rheumatic mitral valve insufficiency, and left ventricular enlargement. The admission diagnoses included infective endocarditis, severe non-rheumatic mitral valve insufficiency, left ventricular enlargement, class IV heart function, acute heart failure, type 2 diabetes mellitus, and chronic HBV carrier status.
Laboratory tests: Blood gas pH 7.50↑, oxygen partial pressure 126 mmHg↑, bicarbonate concentration 30.5 mmol/L↑, white blood cell count 9.44×10^9/L, neutrophil percentage 68.9%, international normalized ratio (INR) 1.05, fibrinogen 6.03 g/L↑, D-dimer quantification 1.61 mg/L, high-sensitivity troponin T 0.006 ng/mL, high-sensitivity troponin I 0.004 ng/mL, erythrocyte sedimentation rate 70 mm/h by the Westergren method, procalcitonin 0.041 ng/mL, N-terminal pro-B-type natriuretic peptide (NT-proBNP) 808 pg/mL↑, glycated hemoglobin (HbA1c) 7.64%↑, anti-SS-A antibody/Ro52KDa weakly positive. Bilateral double sets of blood cultures were drawn immediately upon admission, and the results were negative.
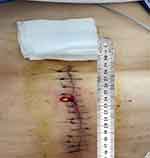
On July 15, the patient underwent mitral valve bioprosthesis replacement and temporary cardiac pacemaker implantation. Postoperative examination on the first day revealed a clean surgical wound dressing. Five days post-surgery, the patient exhibited a low-grade fever, with temperatures ranging between 37.5°C and 38.3°C. Laboratory investigations showed a leukocyte count peaking at 14.15 × 10^9/L and a neutrophil percentage at 91.9%. Procalcitonin (PCT) levels peaked at 1.88 ng/mL, indicating a systemic inflammatory response. Despite negative sputum and blood cultures, the patient was treated with ceftazidime (1g, Q12h) from July 6 to August 30, and vancomycin (1g, Q12h) from July 17 to July 25 for anti-infective therapy. On August 11, the patient reported wound pain and a slight decrease in appetite. On August 13, dark red serous discharge was noted from the upper part of the wound (Figure 1), prompting wound opening for drainage. Subsequent laboratory tests revealed an ultra-sensitive C-reactive protein level of 129.19 mg/L, a leukocyte count of 9.91 × 10^9/L, and a neutrophil percentage of 76.7%. Ceftazidime was continued for anti-infective purposes, and albumin was administered due to hypoalbuminemia (28.7 g/L). On August 21, the patient presented with fat liquefaction at the mid-segment wound, which was managed by draining the liquefied fat and performing bedside debridement and suture. On August 23, bone wax fragments were visible in the wound effusion, and the effusion was sent to the microbiology lab for pathogen culture. On August 23, Gram staining was performed on the wound exudate to identify bacteria and fungi, with no predominant organisms observed. The exudate was cultured on Columbia sheep blood agar and MacConkey agar and incubated at incubator, with daily observations. On the fourth day of incubation, small, rough, white, dry colonies were observed on the Columbia sheep blood agar. The semi-quantitative colony count result was 3+. (Figure 2) Gram staining revealed Gram-positive bacilli under optical microscopy at 1000x magnification,3+ semi-quantitative (Figure 3). Acid-fast staining under similar conditions demonstrated red-stained bacilli,3+ semi-quantitative (Figure 4). The strain was sent to a third-party testing company (Guangzhou KingMed Diagnostics Company Limited) for identification. 16sRNA gene sequencing was employed to identify the colonies cultured on Columbia sheep blood agar, and the result was identified as Mycobacterium porcinum. Additionally. The sequencing results were compared using the BLAST algorithm, showing a 100% similarity to the reference sequence of Mycobacterium porcinum strains.
|
Figure 1 Wound infection manifesting as serosanguineous exudation. |
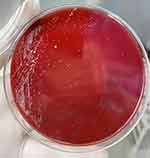 |
Figure 2 After enrichment, the morphology of colonies on Columbia agar plates can be observed after 4 days of cultivation. |
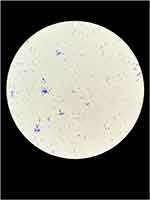 |
Figure 3 Microscopic appearance of Mycobacterium porcinum in pure culture (Gram staining, ×1000). |
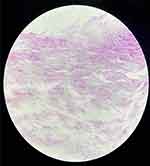 |
Figure 4 Microscopic appearance of Mycobacterium porcinum in pure culture (acid-fast staining, ×1000). |
After learning that the wound was infected with Mycobacterium porcinum, the clinicians, in accordance with the Sanford Guide to Antimicrobial Therapy5 and referring to the treatment regimen for sporadic mycobacterial infections, initiated anti- Mycobacterium porcinum therapy on August 30th. The regimen included a combination of three drugs: amikacin (0.4g,QD), azithromycin (0.5g,QD), and moxifloxacin (0.4g,QD), with a treatment course of at least 1 month. Concurrently, debridement of the wound was performed. After the initiation of the treatment, the white blood cell count was monitored at 11.25×109/L, with a neutrophil percentage of 68.9% and a procalcitonin (PCT) level of 0.053 ng/mL. The wound improved following the antimicrobial therapy, and the patient was discharged.
As shown in Figure 5, the patient was treated with ceftazidime from August 13 to August 30, during which body temperature remained stable However, the white blood cell (WBC) count and neutrophil percentage showed an increasing trend, while high-sensitivity C-reactive protein (hs-CRP) levels remained abnormally elevated. On August 29, the patient suddenly developed chills and high fever, with body temperature spiking to 39.5°C, indicating that ceftazidime was only partially effective in controlling the infection. When the bacterial load increased and disrupted the immune balance, Mycobacterium porcinum triggered a robust immune response. Subsequent clinical evaluation confirmed that M. porcinum infection requires prolonged combination therapy due to its extended recovery period.
 |
Figure 5 The changes in infection indicator levels throughout the patient’s disease course. |
Discussion and Conclusion
Rapidly growing mycobacteria are omnipresent in various environmental niches, exhibiting natural resistance to a spectrum of disinfectants, heavy metals, and antibiotics. This resistance is attributed to their lipid-rich cell walls, which facilitate their survival in aquatic systems. These mycobacteria can endure on both natural and artificial surfaces owing to their capacity to form protective biofilms, shielding them from detrimental external factors.6–10 The biofilm produced by Mycobacterium spp. encapsulates bacterial cells in a sophisticated extracellular matrix, which acts as a barrier against antibiotic penetration. Studies have revealed that certain mycobacteria exhibit susceptibility to antibiotics such as amikacin and clarithromycin in their planktonic state, but their antibiotic activity is significantly diminished when these bacteria reside in a biofilm state.11,12 The mechanisms underlying this reduction in antibiotic efficacy may involve altered metabolic states, activation of resistance genes (eg, inducible methyltransferases), and the formation of persister cells.13–15 Bacteria embedded within biofilms can survive antibiotic treatment and resume proliferation upon cessation of therapy, leading to recurrent infections.16 These mycobacteria can endure on both natural and artificial surfaces owing to their capacity to form protective biofilms, shielding them from detrimental external factors.17
Mycobacterium porcinum, initially isolated from the lymph nodes of pigs exhibiting tuberculous-like infections in 197318 was formerly classified within the Mycobacterium fortuitum group. Schinsky et al19 re-evaluated certain strains previously identified as the third biovariant of Mycobacterium fortuitum using molecular techniques, distinguishing Mycobacterium porcinum from other rhamnose-negative strains within the complex and identifying it as a human pathogen for the first time in 2004. This Gram-positive bacillus exhibits red staining with both acid-fast and weakly acid-fast methods. It is capable of growth on blood culture medium at temperatures of 28°C, 37°C, and 42°C after three days of incubation.20 Detection of Mycobacterium porcinum in tap water samples has been reported.21 Unlike other mycobacterial species, it has not been linked to outbreaks or pseudo-outbreaks.19 Mycobacterium porcinum is known to cause infections involving the skin, soft tissue, respiratory tract, bone marrow, and catheters.20–23
The pathogenesis of Mycobacterium porcinum infection is primarily attributed to cell-mediated immunity and delayed-type hypersensitivity reactions mediated by CD4+ T cells. Various cytokines, including interferon-γ, interleukin-12, and tumor necrosis factor-α, are implicated in the inflammatory response, leading to granuloma formation and potentially resulting in tissue necrosis and cavity formation.24–27 There is evidence in the literature suggesting that the presence of anti-IFN-γ autoantibodies is a significant risk factor for extrapulmonary non-tuberculous mycobacterial (NTM) infections.28
In studies investigating host-microbiome interactions at the transcriptomic level, emerging research has demonstrated that the host may regulate microbial metabolism and immune responses through immunoglobulin-like domain-containing proteins as well as ITIH2, PAEP, and TDRD9 factors.29 Notably, host gene expression has been shown to reciprocally influence microbiota composition, with significant associations observed between specific bacterial abundances and human genomic variations.30 Chronic hyperglycemia in diabetic patients impairs immune function through multiple mechanisms, significantly compromising host defenses against rapidly growing mycobacteria (RGM). The hyperglycemic microenvironment suppresses neutrophil chemotaxis, phagocytic activity, and bactericidal capacity31 while impairing macrophage antigen presentation, thereby reducing pathogen clearance efficiency.32 Furthermore, diabetes disrupts the critical balance between pro-inflammatory (eg,TNF-α,IL-6) and anti-inflammatory cytokines (eg, IL-10), resulting in immune dysregulation that hinders effective RGM elimination.33,34 Notably, T-cell dysfunction – particularly in CD4+ and CD8+ T cell populations – further diminishes immune surveillance and compromises the host’s ability to control RGM infections.35,36
A review of recent case reports on Mycobacterium infections revealed only three documented cases of Mycobacterium porcinum infection, with the remainder involving other mycobacterial species. The literature indicates that the majority of affected patients are middle-aged or elderly individuals, with infections predominantly occurring in immunocompromised hosts with underlying conditions such as diabetes mellitus and end-stage renal disease (ESRD). However, cases have also been reported in immunocompetent hosts following invasive procedures (eg, cosmetic injections). All cases necessitated multidrug regimens, typically including macrolides (eg, clarithromycin), aminoglycosides (eg, amikacin), and fluoroquinolones, with dynamic adjustments based on antimicrobial susceptibility testing. Although clinical improvement was achieved in most patients, treatment failures were observed, such as in cases of catheter-related Mycobacterium abscessus bacteremia. When cultures were negative, molecular diagnostic techniques like 16S rRNA sequencing played a pivotal role in diagnosis and management. Patients with multiple comorbidities who developed disseminated nontuberculous mycobacterial (NTM) infections had poorer prognoses. The patient in this study had diabetes mellitus and underwent cardiac surgery, rendering him immunocompromised and at risk for M. porcinum infection. Prolonged conventional culture incubation increased the detection yield, and 16S rRNA sequencing confirmed the presence of M. porcinum. Combination therapy with amikacin, azithromycin, moxifloxacin, and surgical debridement successfully controlled the infection (Table 1).
 |
Table 1 Review of the Literature on Reported Cases of M. Porcinum Infection and Rapidly Growing Mycobacteria |
Extensive literature review revealed that, to date, approximately 200 species of rapidly growing mycobacteria have been identified.44 Of these, 95% are ubiquitously present in the environment, being found in soil, air45 and water.46 The majority of these mycobacteria are saprophytic or non-pathogenic to humans and animals, with only a minority of species capable of causing infections in animals or humans. The increasing global population age, underlying metabolic diseases, pulmonary conditions,47,48 immunosuppression,49,50 and broad-spectrum antibiotic therapy47,51 are all host factors that predispose individuals to infections caused by nontuberculous mycobacteria (NTM). Given the high abundance of NTM in environmental niches such as soil, natural water sources, and drinking water supplies, there is often a high rate of human-pathogen contact, which in turn leads to an increased incidence of NTM infections.52
In this case report, the patient presented with elevated infection indicators, characterized by red, swollen, and painful wounds, along with tissue necrosis and granuloma formation. Serial cultures of wound secretions consistently detected Mycobacterium porcinum. Following the confirmation of Mycobacterium porcinum infection, the patient was promptly initiated on anti-infective treatment. The clinical management of rapidly growing mycobacterial infections varies slightly depending on the body site affected. This patient was treated with a regimen consisting of amikacin (0.4g, QD), azithromycin (0.5g, QD), and moxifloxacin (0.4g, QD) for anti-infective purposes, concurrent with active surgical debridement to remove foreign bodies. Post-treatment, infection indicators, including leukocyte count, procalcitonin (PCT), and interleukin-6 (IL-6), exhibited a stable downward trend, coincident with symptomatic improvement and overall condition amelioration, thereby validating the treatment efficacy.
Owing to the extended culture time and the inherent difficulty in cultivation, Mycobacterium porcinum is frequently overlooked in clinical settings. To enhance detection, the culture duration for abscesses, wound secretions, and necrotic tissues can be extended to 5 or 7 days, with interim reporting at 3, 5, and 7 days to capture the rapid growth of mycobacteria. In this instance, the specimen only demonstrated 4cfu small colonies on the Columbia sheep blood agar plate on the fourth day of culture. For non-open abscesses, deep wound secretions, and necrotic tissues, pre-enrichment followed by subculturing on Columbia sheep blood agar plates is recommended to augment the positive detection rate. In this case, the laboratory pre-enriched the second specimen on brain heart infusion agar and concurrently inoculated it onto Columbia sheep blood agar plates. After one day of enrichment, subculturing onto Columbia sheep blood agar plates resulted in dense colonies of Mycobacterium porcinum by the third day (Figure 2). This enrichment method is preferable for non-open specimens or deep tissue specimens to prevent the interference of fast-growing colonies with the growth of slow-growing ones, or for simultaneous inoculation on Columbia sheep blood agar plates for comparative growth observation. In cases of recurrent infections where Gram staining smears do not reveal specific bacteria, various staining methods, such as weakly acid-fast and acid-fast staining, can be employed to identify pathogens. It is imperative for the laboratory to establish a communication channel with the clinic, closely liaise to understand the condition, and conduct relevant tests to expedite the identification of the pathogen, facilitating the formulation of an effective anti-infective plan and treatment strategy.
The patient in this case had diabetes mellitus. Diabetic patients frequently exhibit impaired wound healing due to persistent hyperglycemia-induced pathophysiological alterations. Recent advances in biomaterial engineering have developed multifunctional hydrogels capable of modulating the diabetic wound microenvironment through three key mechanisms: immunomodulation, antimicrobial activity, and tissue regeneration. Naturally derived dual dynamic crosslinked hydrogels4 demonstrate significant potential in reprogramming macrophage phenotypes from pro-inflammatory M1 to anti-inflammatory M2, thereby attenuating chronic inflammation and promoting physiological healing. For instance, obakunone-incorporated hydrogels facilitate the critical transition from the inflammatory to proliferative phase, while protocatechualdehyde (PA) and ferric ion (Fe³+)-loaded systems53 provide synergistic antibacterial effects via sustained drug release. Additionally, dynamically crosslinked self-healing hydrogels enable sequential delivery of antimicrobial agents and growth factors, optimizing the temporal requirements of wound repair. Furthermore, collagen/hyaluronic acid-based hydrogels releasing sulfated hyaluronan54 have been shown to improve diabetic wound healing by modulating inflammatory macrophage activity and providing extracellular matrix-mimetic support. These advanced hydrogel systems represent a promising therapeutic strategy for refractory diabetic wounds, offering targeted immunomodulation, enhanced antimicrobial efficacy, and controlled regenerative factor delivery while minimizing systemic side effects. Further clinical studies are warranted to establish standardized treatment protocols for their translation into routine practice.
For established cavitary and lumenal infections, emerging research has demonstrated the potential of nature-inspired magnetically actuated microrobots to penetrate infected sites. These microdevices utilize low-frequency magnetic fields to perform localized biofilm disruption through mechanical scraping action while enabling targeted antimicrobial delivery.55 Furthermore, advanced systems incorporating high-frequency alternating magnetic fields can achieve precise thermal ablation of bacterial biofilms via controlled heating of low-friction soft robotic platforms. Complementary approaches include self-propelled microrocket systems that utilize chemically generated bubble propulsion to actively penetrate gastrointestinal biofilms and enhance drug delivery efficacy.56 Notably, recent developments in magnetic living hydrogel composites demonstrate multifunctional capabilities for intestinal infection management. These biohybrid systems combine magnetic navigation for precise localization with controlled therapeutic release and diagnostic functions, offering a comprehensive theranostic platform.57 These innovative technologies represent a paradigm shift in the treatment of complex cavitary infections by combining mechanical biofilm disruption with spatially controlled therapeutic delivery.
The present study highlights the critical importance of optimizing culture strategies to improve the detection of Mycobacterium porcinum, a slow-growing yet easily overlooked pathogen in chronic wound infections, particularly in diabetic patients. By extending incubation periods to 7 days with interim reporting at 3, 5, and 7 days, alongside selective enrichment in Brain Heart Infusion Broth for deep or non-open wounds, we significantly enhanced recovery rates, reducing diagnostic delays. Furthermore, integrating alternative staining methods (weak acid-fast, Ziehl-Neelsen) proved valuable when conventional Gram staining failed to identify pathogens. Beyond diagnostics, emerging therapies such as immunomodulatory hydrogels—engineered to shift macrophage polarization from pro-inflammatory M1 to reparative M2 phenotypes—demonstrate promise in disrupting the chronic inflammatory milieu of diabetic wounds. Innovations like dynamic crosslinked hydrogels enabling sequential drug/growth factor release, or magnetically guided microrobots for biofilm penetration and targeted antimicrobial delivery, represent transformative approaches for resistant infections. Future efforts should prioritize rapid molecular diagnostics, clinically adaptable biomaterials, and interdisciplinary strategies bridging microbiology, nanotechnology, and immunology to address the multifaceted challenges of chronic wound management.
Ethics Approval and Consent for Publication
This study has been reviewed and approved by the Research Ethics Committee of Shenzhen Hospital of Fuwai Hospital, Chinese Academy of Medical Sciences. The patient provided informed consent for publication of the clinical details and written informed consent was obtained. Written informed consent was provided by the patient for the publication of the case details and images. Details of the case can be published without institutional approval.
Acknowledgment
We are grateful for the support and dedication of all staff at the Department of Laboratory Medicine, Pharmacy, and Internal Medicine at the Shenzhen Hospital of Fuwai Hospital, Chinese Academy of Medical Sciences.
Funding
Supported by Shenzhen Clinical Research Center for Cardiovascular Diseases Fund (No. 20220819165348002).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barbara A, Brown-Elliot RJ. Mycobacterium: clinical and Laboratory Characteristics of Rapidly Growing Mycobacteria. 2024.
2. Falkinham JO. Surrounded by mycobacteria: nontuberculous mycobacteria in the human environment. J Appl Microbiol. 2009;107(3):356–367. doi:10.1111/j.1365-2672.2009.04161.x
3. Newton JA Jr, Weiss PJ, Bowler WA, et al. Soft-tissue infection due to mycobacterium smegmatis: report of two cases. Clin Infect Dis. 1993;16(4):531–533. doi:10.1093/clind/16.4.531
4. Shi T, Lu H, Yang LG. Naturally derived dual dynamic crosslinked multifunctional hydrogel for diabetic wound healing. Composites Part B Eng. 2023;257:110687. doi:10.1016/j.compositesb.2023.110687
5. Gilbert D, Moellering R, Sande M. The Sanford guide to antimicrobial therapy. 2004.
6. Russell AD, Hammond SA, Morgan JR. Bacterial resistance to antiseptics and disinfectants. J Hosp Infect. 1986;7(3):213–225. doi:10.1016/0195-6701(86)90071-X
7. Russell AD. Activity of biocides against mycobacteria. Soc Appl Bacteriol Symp Ser. 1996;25:87S–101S. doi:10.1111/j.1365-2672.1996.tb04837.x
8. Selvaraju SB, Khan IUH, Yadav JS. Biocidal activity of formaldehyde and nonformaldehyde biocides toward mycobacterium immunogenum and pseudomonas fluorescens in pure and mixed suspensions in synthetic metalworking fluid and saline. Appl Environ Microbiol. 2005;71(1):542–546. doi:10.1128/AEM.71.1.542-546.2005
9. Carson L, Favero GM, Petersen LN, et al. Growth characteristics of atypical mycobacteria in water and their comparative resistance to disinfectants. Appl Environ Microbiol. 1978;36(6):839. doi:10.1007/BF00394328
10. Smithwick RW, Stratigos CB, David HL. Use of cetylpyridinium chloride and sodium chloride for the decontamination of sputum specimens that are transported to the laboratory for the isolation of Mycobacterium tuberculosis. J Clin Microbiol. 1975;1(5):411–413. doi:10.1128/JCM.1.5.411-413.1975
11. Greendyke R, Byrd TF. Differential antibiotic susceptibility of Mycobacterium abscessus variants in biofilms and macrophages compared to that of planktonic bacteria. Antimicrob Agents Chemother. 2008;52(6):2019–2026. doi:10.1128/AAC.00986-07
12. Ortíz-Pérez A, Martín-de-Hijas N, Alonso-Rodríguez N, et al. Importance of antibiotic penetration in the antimicrobial resistance of biofilm formed by non-pigmented rapidly growing mycobacteria against amikacin, ciprofloxacin and clarithromycin. Enferm Infecc Microbiol Clin. 2011;29(2):79–84. doi:10.1016/j.eimc.2010.08.016
13. Anderson GG, O’Toole GA. Innate and induced resistance mechanisms of bacterial biofilms. Curr Top Microbiol Immunol. 2008;322:85–105. doi:10.1007/978-3-540-75418-3_5
14. Lewis K. Multidrug Tolerance of Biofilms and Persister Cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi:10.1080/00222930400025896
15. Kester JC, Fortune SM. Persisters and beyond: mechanisms of phenotypic drug resistance and drug tolerance in bacteria. Crit Rev Biochem Mol Biol. 2014;49(2):91–101. doi:10.3109/10409238.2013.869543
16. Hall-Stoodley L, Paul S, Sandeep K, et al. Towards diagnostic guidelines for biofilm-associated infections. FEMS Immunol Med Microbiol. 2012;66(2):127–145. doi:10.1111/j.1574-695X.2012.00968.x
17. Wallace RJ, Brown BA, Onyi GO. Susceptibilities of Mycobacterium fortuitum biovar. fortuitum and the two subgroups of Mycobacterium chelonae to imipenem, cefmetazole, cefoxitin, and amoxicillin-clavulanic acid. Antimicrob Agents Chemother. 1991;35(4):773–775. doi:10.1128/AAC.35.4.773
18. Tsukamura M, Nemoto H, Yugi H. Mycobacterium porcinum sp. nov. a porcine pathogen. Int J Syst Bacteriol. 1983;33(2):162–165. doi:10.1099/00207713-33-2-162
19. Schinsky MF, Morey RE, Steigerwalt AG. Taxonomic variation in the Mycobacterium fortuitum third biovariant complex: description of Mycobacterium boenickei sp. nov. Mycobacterium houstonense sp. nov. Mycobacterium neworleansense sp. nov. and Mycobacterium brisbanense sp. nov. Int J Syst Evol Microbiol. 2004;54:1653–1667. doi:10.1099/ijs.0.02743-0
20. Wallace RJ, Brown-Elliott BA, Wilson RW. Clinical and laboratory features of Mycobacterium porcinum. J Clin Microbiol. 2005;42(12):5689–5697. doi:10.1128/JCM.42.12.5689-5697.2004
21. Brown-Elliott BA, Wallace RJ, Tichindelean C, et al. Five-year outbreak of community- and hospital-acquired Mycobacterium porcinum infections related to public water supplies. J Clin Microbiol. 2011;49(12):4231–4238. doi:10.1128/JCM.05122-11
22. Paul GR, Leber A, Nemastil CJ, et al. Identification of Mycobacterium porcinum in patients with cystic fibrosis: pathogen or contaminant? J Cyst Fibros. 2020;19(4):580–586. doi:10.1016/j.jcf.2020.01.004
23. Bouam A, Levasseur A, Drancourt M. Draft genome sequence of Mycobacterium porcinum CSURP1564. Genome Announc. 2018;6(16):e00291–18. doi:10.1128/genomea.00291-18
24. Daley CL, Iaccarino JM, Lange C, et al. Treatment of nontuberculous mycobacterial pulmonary disease: an official ATS/ERS/ESCMID/IDSA clinical practice guideline. Eur Respir J. 2020;56(1):2000535. doi:10.1183/13993003.00535-2020
25. Lin HC, Chen CJ, Lin CS, et al. NK cell-derived IFN-γ protects against nontuberculous Mycobacterial lung infection. J Immunol. 2018;201(1):147–158. doi:10.4049/jimmunol.1800123
26. Tang S. Expert consensus on the diagnosis and treatment of non-tuberculous mycobacterial disease. Chin J Tuberc Respir Dis. 2012;2012:1.
27. Tang S, Gao W, Chen C, et al. Clinical Tuberculosis. People’s Medical Publishing House; 2011.
28. Kham-Ngam I, Ploenchan C, Ananta P, et al. Epidemiology of and risk factors for extrapulmonary nontuberculous mycobacterial infections in Northeast Thailand. PeerJ. 2018;6:e5479. doi:10.7717/peerj.5479
29. Huang L, Luo S, Liu S, et al. Comparative multionics analyses reveal the breed effect on the colonic host-microbe interactions in pig. iMetaOmics. 2024;1:e8. doi:10.1002/imo2.8
30. Boccuto L, Tack J, Ianiro G, et al. Human genes involved in the interaction between host and gut microbiome: regulation and pathogenic mechanisms. Genes. 2023;14(4):857. doi:10.3390/genes14040857
31. Dowey R, Iqbal A, Heller SR, et al. A bittersweet response to infection in diabetes; targeting neutrophils to modify inflammation and improve host immunity. Front Immunol. 2021;12:678771. doi:10.3389/fimmu.2021.678771
32. Lopez-Lopez N, Martinez AGR, Garcia-Hernandez MH. Type-2 diabetes alters the basal phenotype of human macrophages and diminishes their capacity to respond, internalise, and control Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. 2018;113. doi:10.1590/0074-02760170326
33. Nathella PK, Kumar NP, George PJ, et al. Impaired cytokine but enhanced cytotoxic marker expression In Mycobacterium tuberculosis –induced CD8 + T cells in individuals with type 2 diabetes and latent Mycobacterium tuberculosis infection. J Infect Dis. 2016;213(5):866–870. doi:10.1093/infdis/jiv484
34. Ponnana M, Sivangala R, Joshi L, et al. IL-6 and IL-18 cytokine gene variants of pulmonary tuberculosis patients with co-morbid diabetes mellitus and their household contacts in Hyderabad. Gene. 2017;627:298–306. doi:10.1016/j.gene.2017.06.046
35. Kumar NP, Moideen K, Dhakshinraj SD, et al. Profiling leucocyte subsets in tuberculosis-diabetes co‐morbidity. Immunology. 2015;146(2):243–250. doi:10.1111/imm.12496
36. Kathamuthu GR, Kumar NP, Moideen K, et al. Decreased frequencies of gamma/delta T cells expressing Th1/Th17 cytokine, cytotoxic, and immune markers in latent tuberculosis-diabetes/pre-diabetes comorbidity. Front Cell Infect Microbiol. 2021;11:756854. doi:10.3389/fcimb.2021.756854
37. Shah S, Zaidi K, Onyia W. Mycobacterium porcinum disseminated infection in non-severely immunocompromised host. Cureus. 2024;16(3):e55889. doi:10.7759/cureus.55889
38. Wei WJ, Luo R, Chen ZK, He JB. Mycobacterium porcinum infection of hilar and mediastinal lymph nodes: a case report and literature review. Infect Drug Resist. 2023;16:1–7. doi:10.2147/IDR.S432987
39. Schenfeld L, Patil T, Schenfeld L, et al. Mycobacterium porcinum peritonitis in a patient on continuous ambulatory peritoneal dialysis. J Gen Intern Med. 2010;26(3):346–348. doi:10.1007/s11606-010-1571-y
40. Dash A, Gupta N, Ray Y, et al. Choosing the therapy for neurological infection with rapidly growing mycobacteria. Drug Discov Ther. 2020;14(4):211–212. doi:10.5582/ddt.2020.03026
41. Pan SF, Zhang YY, Wang XZ, et al. Catheter-related infections caused by Mycobacterium abscessus in a patient with motor neurone disease: a case report. World J Clin Cases. 2022;10(15):5082–5087. doi:10.12998/wjcc.v10.i15.5082
42. Tan YZ, Yuan T, Tan L, et al. Lumbar infection caused by Mycobacterium paragordonae: a case report. World J Clin Cases. 2021;29(9):8879.
43. Deng L, Luo YZ, Liu F, et al. Subcutaneous infection caused by Mycobacterium abscessus following cosmetic injections of botulinum toxin: a case report. World J Clin Cases. 2022;18(10):6141.
44. Tortoli E, Fedrizzi T, Meehan CJ, et al. The new phylogeny of the genus Mycobacterium: the old and the news. Infect Genet Evol. 2017:56. doi:10.1016/j.meegid.2017.10.013
45. Angenent LT, Kelley ST, Amand AS, et al. Molecular identification of potential pathogens in water and air of a hospital therapy pool. Proc Natl Acad Sci U S A. 2005;102(13):4860–4865. doi:10.1073/pnas.0501235102
46. Falkinham JO III, Iseman MD, Haas PD, et al. Mycobacterium avium in a shower linked to pulmonary disease. J Water Health. 2008;6(2):209–213. doi:10.2166/wh.2008.032
47. Roux A-L, Catherinot E, Ripoll F. Multicenter study of prevalence of Nontuberculous Mycobacteria in patients with cystic fibrosis in France. J Clin Microbiol. 2009;47(12):4124–4128. doi:10.1128/JCM.01257-09
48. Olivier KN, Weber DJ, Wallace RJ, et al. Nontuberculous mycobacteria. I: multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med. 2003;167(6):828–834. doi:10.1164/rccm.200207-678OC
49. Collins FM. AIDS-related mycobacterial disease. Springer Semin Immunopathol. 1988;10(4):375–391. doi:10.1007/bf02053847
50. Collins FM. Mycobacterial disease, immunosuppression, and acquired immunodeficiency syndrome. Clin Microbiol Rev. 1989;2(4):360–377. doi:10.1128/CMR.2.4.360
51. Catherinot E, Roux AL, Vibet MA, et al. Mycobacterium avium and Mycobacterium abscessus complex target distinct cystic fibrosis patient subpopulations. J Cyst Fibros. 2013;12(1):74–80. doi:10.1016/j.jcf.2012.06.009
52. Falkinham JO. Environmental sources of Nontuberculous Mycobacteria. Clin Chest Med. 2015;36(1):35–41. doi:10.1016/j.ccm.2014.10.003
53. Chen AM, Tang JY, Li Y, et al. Dynamic covalent constructed self-healing hydrogel for sequential delivery of antibacterial agent and growth factor in wound healing. Chem Eng J. 2019;373:413–424. doi:10.1016/j.cej.2019.05.043
54. Hauck S, Zager P, Halfter N, et al. Collagen/hyaluronan based hydrogels releasing sulfated hyaluronan improve dermal wound healing in diabetic mice via reducing inflammatory macrophage activity. Bioact Mater. 2021;6:434–447. doi:10.1016/j.bioactmat.2021.04.026
55. Wang B, Chen Y, Ye Z, et al. Low-friction soft robots for targeted bacterial infection treatment in gastrointestinal tract. Cyborg Bionic Syst. 2024;5:0138. doi:10.34133/cbsystems.0138
56. Mundaca-Uribe R, Askarinam N, Fang RH, et al. Towards multifunctional robotic pills. Nat Biomed Eng. 2024;8(11):1334–1346. doi:10.1038/s41551-023-01090-6
57. Liu X, Yang Y, Inda ME, et al. Magnetic living hydrogels for intestinal localization, retention, and diagnosis. Adv Funct Mater. 2021. doi:10.1002/adfm.202010918



