Mechanisms and Nanomedicine Interventions of Acute Lung Injury Induced by Intestinal Ischemia-Reperfusion: A Mini Review
Introduction
Intestinal ischemia-reperfusion (II/R) injury is a common organ injury in the perioperative period and is commonly seen in severe infections, trauma, shock, and during a variety of surgical procedures, such as extracorporeal circulation, intestinal obstruction, small bowel transplantation, and abdominal aortic aneurysm surgery.1 II/R not only causes local damage to the intestinal tract but also the destruction of the intestinal mucosal barrier, leading to the displacement of a large number of bacteria and endotoxins from the intestines to the outside of the intestines, which triggers systemic inflammatory response syndrome (SIRS) and multi-organ failure, with a mortality rate as high as 60%-80%.2 In the early stages of II/R, insufficient intestinal perfusion is often the primary trigger, typically resulting from a sudden decline in cardiac output, which can be detected using patient monitoring devices. In patients with a worsening overall condition, characterized by paralytic ileus and rectal bleeding, CT imaging can further confirm the presence of non-occlusive mesenteric ischemia (NOMI).3 This phenomenon is commonly observed in intensive care unit settings and is recognized as the initiating event of the II/R cascade. During II/R, intestinal tissues undergo ischemic injury due to hypoxia and lack of energetic substances, followed by metabolic disorders caused by reperfusion, activation of inflammatory signaling pathways, and oxidative stress pathways, which ultimately lead to cell damage and death.4 Reactive oxygen species (ROS) as well as inflammatory factors produced during the II/R process rapidly activate immune cells, triggering SIRS.
During II/R, the lungs, as the only organ receiving full cardiac output, are particularly sensitive to the damaging blows of circulating inflammatory cells and inflammatory mediators, and often become one of the earliest distal organs to be involved.5 Acute lung injury (ALI) is a common distal organ complication of II/R, with a very high morbidity and mortality rate.6 The pathological manifestations of ALI include destruction of the alveolar epithelium and pulmonary capillary endothelium, alveolar edema, neutrophil infiltration, and hyaline membrane formation.7 The mechanism of ALI is complex and involves multiple interrelated pathophysiological processes such as inflammatory response, oxidative stress, apoptosis, autophagy, and ferroptosis.8 Due to the over-activation of immune cells, the inflammatory response is out of control, forming a storm of inflammatory factors, resulting in damage to alveolar epithelial cells and peri-alveolar capillary endothelial cells, followed by diffuse interstitial and alveolar edema, with clinical manifestations of hypoxemia and acute respiratory insufficiency, and in severe cases, it can be further developed into acute respiratory distress syndrome (ARDS), which will ultimately lead to respiratory failure or even death.8,9 To date, the etiology and pathogenesis of II/R-ALI have not been fully elucidated, and there is a lack of effective therapeutic measures, so there is an urgent need to find safer and more effective treatments.
The rapid development of nanomedicine has brought light to this challenge. Nanoparticles, generally defined as particles with diameters between 1 and 100 nanometers, are widely used in various fields due to their unique properties.10 While this range is commonly referenced, particles larger than 100 nm can also be functional depending on the biological context and delivery route. Nanomedicines can enhance drug efficacy and reduce side effects by virtue of their small particle size, high bioavailability, and targeting.11 By changing the size, morphology, and surface chemical groups of nanoparticles, their biodistribution can be enhanced to achieve precise targeting of specific tissues and optimize therapeutic effects.12 In the treatment of ALI, nanomaterials have demonstrated significant efficacy by inhibiting inflammation, antioxidant and reducing cellular damage.13 Nanomedicine offers new strategies to overcome the limitations of conventional drug therapy, including reducing systemic toxicity and improving targeted drug delivery.14 However, the clinical translation of nanomedicines remains challenging. Ensuring reproducible and consistent therapeutic efficacy across different patient populations is a major obstacle.15 Many nanomedicines exhibit excellent preclinical results in animal models, but their efficacy often fails to translate into comparable clinical benefits in humans due to complex biological barriers, heterogeneous tumor microenvironments, or differences in metabolism and immune responses.16 In this review, we summarize the major pathological mechanisms involved in II/R-ALI and highlight the current progress in nanomedicine-based therapeutic strategies.
Mechanisms of Acute Lung Injury Induced by Intestinal Ischemia-Reperfusion
II/R refers to the phenomenon where damage worsens upon restoration of blood flow to the intestine after ischemia. The gastrointestinal tract is involved in digestion, absorption, metabolism, endocrine, and immune functions, with the mucosal barrier crucial for maintaining homeostasis.17 II/R commonly occurs in acute hemorrhage, shock, disseminated intravascular coagulation, intestinal obstruction, multiple traumas, organ transplantation surgeries, and cardiopulmonary bypass.1
II/R damages not only the intestine but also distant organs like the lungs, heart, liver, and kidneys.18 Among these, ALI often appears first and is most common in multiple organ dysfunction syndrome (MODS).19 Prolonged reperfusion increases pulmonary capillary permeability, extensively damages the pulmonary vascular endothelium and alveolar epithelium, damages the basement membrane, thickens the alveolar wall, causes significant interstitial and alveolar edema, and results in massive neutrophil infiltration with cytokine-rich proteinaceous edema fluid. Ultimately, this leads to lung dysfunction or respiratory failure, progressing to ARDS.20,21 This complex mechanism underscores the critical importance of studying and managing II/R-ALI (Figure 1).
Oxidative Stress Response
One of the primary mechanisms contributing to ALI in the context of II/R is oxidative stress.22 ROS, mainly produced by mitochondria, are key participants and markers of oxidative stress. During II/R, a large amount of ROS is generated, becoming a key factor in triggering ALI.23
Under ischemic conditions, xanthine dehydrogenase converts to xanthine oxidase, leading to the massive generation of ROS.24 During ischemia, calcium overload and ATP degradation increase xanthine dehydrogenase activity. This catalyzes the production of copious levels of ROS in the presence of oxygen molecules upon reperfusion. Furthermore, the formation of ROS is increased by neutrophil respiratory burst, mitochondrial injury, and cytokines including IL-1 and tumor necrosis factor-α (TNF-α).25 In addition to causing tissue damage through direct oxidative injury to cell membranes, lipid peroxidation, and extracellular matrix degradation, ROS also function as important signaling molecules that activate multiple intracellular signaling pathways.26,27 ROS activates NF-κB and MAPK pathways, promoting the expression of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6, thereby amplifying inflammatory responses.28 Moreover, ROS serve as critical triggers for NLRP3 inflammasome activation, facilitating the maturation and release of pro-inflammatory cytokines and inducing inflammatory cell death.29 Additionally, ROS induce loss of mitochondrial membrane potential, promoting the release of pro-apoptotic proteins and activating the mitochondria-dependent apoptotic pathway, leading to programmed cell death.30
Oxidative stress plays a key role in ALI. Excessive ROS compromise the integrity of tight junctions within the alveolar barrier, thereby enhancing pulmonary capillary permeability, facilitating neutrophil infiltration, and promoting the secretion of cytotoxic substances.31 Along with aggravating tissue damage and pulmonary edema, ROS can break down tight junctions in lung tissue and upregulate pro-inflammatory cytokines and chemokines.32 Furthermore, the accumulation of ROS can induce the activation of matrix metalloproteinases (MMPs), particularly MMP-2 and MMP-9, which degrade collagen and elastin in the pulmonary extracellular matrix (ECM), disrupt the integrity of the alveolar-capillary barrier, and further exacerbate pulmonary edema and lung tissue injury.33,34 This suggests that MMPs, as one of the downstream effectors of oxidative stress, play an amplifying role in tissue injury during the progression of II/R-ALI.
Studies show that regulating antioxidant pathways can reduce ROS production and eliminate ROS in the body, an important approach to preventing and treating II/R injury. Oxidative stress-induced apoptosis can be prevented by controlling the PI3K/Akt signaling pathway.35 In ischemia-reperfusion injury, PTEN, which negatively regulates the PI3K/Akt pathway, is elevated. This results in reduced Nrf2 activity and worsened oxidative stress, which compromises intestine and lung function.36
Cytokines and Inflammatory Response
ALI induced by II/R is closely related to cytokine production and release.37 Cytokines are small soluble proteins that transmit signals between cells, influencing cellular behavior and function.38 Cytokines are categorized into pro-inflammatory and anti-inflammatory types according to their roles in the inflammatory process. The imbalance between these responses plays a crucial role in the development of ALI.39
During II/R, ischemia compromises the intestinal mucosal barrier, facilitating the translocation of bacteria and endotoxins into the bloodstream.18 Endotoxins bind to lipopolysaccharide-binding protein in the blood and to CD14 receptors on monocytes/macrophages, activating the monocyte-macrophage system and triggering an inflammatory cascade of mediators and cytokines.40 These inflammatory mediators and cytokines form a complex network that regulates and plays important roles in the pathological processes of inflammation, immunity, and MODS. Key pro-inflammatory cytokines include TNF-α and a range of interleukins, including IL-1, IL-6, and IL-8.41 TNF-α produced by intestinal macrophages plays a key role in MODS development, while IL-8 induces neutrophil deformation and degranulation, releasing lysosomal enzymes and ROS, further exacerbating tissue damage.42
Massive ROS production, calcium overload, and neutrophil aggregation are also important mechanisms of II/R injury.43 After the intestinal barrier is damaged, many bacteria and endotoxins enter the circulatory system, activating the TLR4 signaling pathway and triggering an inflammatory response.44 TLR4 binds to its ligand and activates various signaling pathways, such as the NF-κB pathway, which results in the generation of pro-inflammatory cytokines and exacerbates lung injury.45 NF-κB, an important nuclear transcription factor, regulates essential cellular signaling pathways. When the intestinal mucosal barrier is disrupted during II/R, a large amount of bacteria, endotoxins, and inflammatory mediators enter the bloodstream and activate the NF-κB signaling pathway. This increases the expression of IL-1, IL-6, and TNF-α in lung tissue, promotes neutrophil infiltration, and causes an inflammatory response in lung tissue.46
Additionally, activation of the NLRP3 inflammasome is a key mechanism by which II/R induces ALI.47 The NLRP3 inflammasome detects damage-associated molecular patterns released by both pathogens and the host, subsequently activating caspase-1. This activation leads to the maturation of pro-IL-1β and pro-IL-18, initiating an acute inflammatory response and contributing to oxidative stress.48 Overactivated NLRP3 inflammasomes can also trigger pyroptosis, exacerbating lung tissue damage.49
Notably, MMPs also play a crucial role in the inflammatory response. Studies have shown that the expression of MMP-1, MMP-2, MMP-8, and MMP-9 is significantly upregulated in both acute and chronic pulmonary inflammation. MMPs participate in the pathogenesis and progression of ALI by modulating inflammatory cell infiltration, cytokine release, and degradation of the ECM.33,50,51 For example, MMP-9 facilitates the transmigration of neutrophils across the basement membrane into lung tissue, thereby intensifying the inflammatory response.52,53 The expression of MMPs is induced by inflammatory cytokines such as TNF-α and IL-1β, and their enzymatic activity amplifies the inflammatory cascade, forming a positive feedback loop between inflammation and protease activity, which further aggravates lung tissue injury.54,55
Apoptosis
Apoptosis, a type of programmed cell death, is distinguished by cell shrinkage, membrane blebbing, chromatin condensation, and nuclear fragmentation.56 Typically, intestinal epithelial cells maintain homeostasis through spontaneous apoptosis.57 During II/R, excessive apoptosis increases intestinal permeability, leading to bacterial translocation and apoptosis in distant lung tissues.58
Lung epithelial cells exhibit the activation of the death receptor Fas and its ligand FasL in the acute inflammatory response brought on by II/R.59 Furthermore, the extrinsic apoptosis pathway is started by tumor necrosis factor receptor-1 binding to its ligands, TNF-α and TNF-related apoptosis-inducing ligands.60 The death-inducing signaling complex is formed, adaptor proteins including the Fas-associated death domain are recruited, and caspase-8 and its downstream apoptotic proteins are activated.61 The mitochondrial pathway is principally responsible for mediating intrinsic apoptosis. When cytochrome c is liberated from mitochondria and enters the cytoplasm during II/R, it activates caspase-3, which is reliant on caspase-9, and starts the subsequent apoptotic cascade.62
Additionally, apoptosis is promoted by a reduction in the Bcl-2/Bax protein expression ratio.63 Bcl-2 exerts anti-apoptotic effects by inhibiting mitochondrial permeability changes, reducing ROS production, and regulating intracellular calcium balance.64 In contrast, Bax is a pro-apoptotic gene, and the Bcl-2/Bax ratio determines cell survival.65
One of the ShcA family’s members, p66Shc, is essential to apoptosis. Following II/R, lung tissues exhibit a considerable activation of protein kinase C-β (PKC-β), which upregulates the expression of p66Shc and increases sensitivity to oxidative stress.66 PKC-β-mediated serine phosphorylation of p66Shc promotes its translocation to mitochondria, causing mitochondrial dysfunction and promoting apoptosis. The PKC-βII specific inhibitor LY333531 significantly inhibits p66Shc activation and reduces II/R-ALI.67 Pin1 is an upstream regulator of the p66Shc pathway. Increased Pin1 expression and enzymatic activity post-II/R promote p66Shc mitochondrial translocation and accelerate apoptosis.68 The Pin1 inhibitor juglone can alleviate secondary lung injury and improve survival rates after II/R.
Ferroptosis
Different from classic apoptosis and necrosis, ferroptosis is a kind of cell death marked by an iron reliance and non-apoptotic, caspase-independent cell death.69 Ferroptosis is characterized by the build-up of lipid ROS and intracellular iron, mostly in the mitochondria, which leads to the loss or disappearance of mitochondrial cristae, an increase in membrane density, and the rupture of the outer membrane, which results in cell malfunction.70 Oxidative imbalance is exacerbated by ferroptosis when intracellular antioxidants like glutathione are deficient or the lipid repair enzyme GPx4 is inactivated.71
During II/R, oxidative stress and iron accumulation significantly increase, activating ferroptosis. This is characterized by the downregulation of GPX4, xCT, and FTH1, and the upregulation of pro-ferroptotic factors COX-2 and ACSL4.72 Early ferroptosis triggers an excessive inflammatory response, further exacerbating the lung injury. The interaction between ferroptosis and the inflammatory response is significant. Oxidative stress induced by ferroptosis stimulates the release of inflammatory factors IL-6, TNF-α, and IL-1β, which in turn exacerbate ferroptosis, forming a vicious cycle.22 Ferroptosis inhibitors like deferoxamine can reduce inflammatory responses and alleviate lung injury.72
To control cellular antioxidant responses, nuclear factor E2-related factor 2 (Nrf2) is essential. Nrf2 inhibits ferroptosis by upregulating SLC7A11 and heme oxygenase-1, protecting cells from oxidative damage.73 The apoptosis-stimulating protein of p53 (iASPP) promotes Nrf2 accumulation and nuclear translocation in the cytoplasm through a p53-independent mechanism. As a result, cells are protected against ferroptosis by increased expression of hypoxia-inducible factor-1α (HIF-1α).23 However, HIF-1α promotes ferroptosis under hypoxic conditions, and inhibiting HIF-1α can reduce ferroptosis’ impact on ALI.22 Thus, more research is needed to understand how Hif-1α contributes to II/R-induced ferroptosis in lung tissue cells.
Pyroptosis
During II/R, ischemia-reperfusion creates a lot of ROS, which causes protein misfolding and activates the NLRP3 inflammasome.74 The NLRP3 inflammasome recognizes pathogen- and host-derived damage-associated molecular patterns, promoting its assembly and activation.75 NLRP3 inflammasomes activate caspase-1, which cleaves GSDMD to produce the N-terminal fragment, resulting in pyroptosis. During pyroptosis, cell membrane rupture leads to the massive release of inflammatory cytokines such as IL-1β and TNF-α. These factors induce and exacerbate pulmonary inflammatory responses, increase vascular permeability, and cause vascular and interstitial lung edema.76 This inflammatory response is not limited to the lungs but also affects other distant organs, aggravating overall pathological damage.
Experimental studies show that NLRP3-deficient mice exhibit significantly reduced ALI symptoms post-II/R compared to wild-type mice.77 This is evidenced by reduced inflammatory cell infiltration in lung tissues, alleviated alveolar septal thickening, reduced vascular congestion, and decreased lung edema. The lung tissues of animals lacking NLRP3 exhibit a considerable reduction in the production of TNF-α and IL-1β, indicating the crucial role of the NLRP3 inflammasome in II/R-ALI. Inhibiting NLRP3 inflammasome activation can significantly reduce II/R-induced lung injury. Using antioxidants to inhibit ROS production reduces NLRP3 inflammasome activation, thereby decreasing GSDMD-mediated pyroptosis and the release of inflammatory cytokines, alleviating pulmonary inflammation and injury.78
Autophagy
Autophagy acts in a dual role in II/R-ALI. Moderate autophagy protects lung tissue by clearing damaged cells and regulating immune responses.79 Excessive autophagy may exacerbate lung injury through mechanisms such as activating macrophage apoptosis.80
In their II/R model, Jiang et al observed autophagy dysfunction and atypical activation of intraepithelial lymphocytes (IELs). Enhancing the expression of autophagy-related genes Beclin-1 and Atg16, and stimulating the NOD2/Beclin-1 pathway, augments the autophagy capacity of IELs. This process diminishes the inflammatory response in the intestinal mucosal epithelium, sustains energy balance and cellular homeostasis during ischemia, and consequently ameliorates ALI.81 Another study indicates that II/R generates significant levels of complement C5a in lung tissues. C5a binds to C5a receptors on alveolar macrophages, initiating downstream signaling, promoting and activating macrophage autophagy, ultimately leading to alveolar macrophage apoptosis and disrupting lung homeostasis.82 Therefore, regulating autophagy levels and maintaining them within a moderate range is crucial for mitigating II/R-ALI.
Traditional Treatment Strategies for Intestinal Ischemia-Reperfusion Induced Acute Lung Injury
ALI is a severe clinical syndrome triggered by various factors, including sepsis, infection, trauma, and ischemia-reperfusion, with its most critical manifestation being ARDS.83 The pathophysiological hallmarks of ALI include damage to alveolar epithelial and pulmonary microvascular endothelial cells, increased alveolar-capillary permeability, massive infiltration of inflammatory cells, and pulmonary edema, ultimately leading to ventilation-perfusion mismatch and progressive hypoxemia.84 Among the many causes of ALI, II/R is recognized as a major trigger of remote organ damage, with the lungs being particularly vulnerable.85 During II/R, excessive inflammatory responses and oxidative stress not only disrupt pulmonary structural integrity but also contribute to irreversible respiratory dysfunction and even fibrosis. Due to the complexity of its pathogenesis, no specific treatments are currently available, and clinical management of II/R-ALI still relies primarily on controlling inflammation, reducing oxidative damage, and improving pulmonary function.85
ALI caused by II/R is a complex pathological process requiring the comprehensive application of multiple treatment strategies. Pharmacotherapy primarily involves anti-inflammatory, antioxidant, and anti-apoptotic drugs. Glucocorticoids (eg, dexamethasone, methylprednisolone) effectively reduce lung inflammation by inhibiting NF-κB signaling and pro-inflammatory cytokine release, thereby decreasing alveolar-capillary permeability and pulmonary edema.86,87 Similarly, statins (eg, atorvastatin, pravastatin) stabilize endothelial function and reduce inflammatory responses.88,89 Neutrophil elastase inhibitors (eg, sivelestat) protect lung tissue by limiting neutrophil-mediated damage and oxidative stress, showing potential to reduce mechanical ventilation duration and ICU stay.90 Sivelestat, which was rapidly approved during the COVID-19 pandemic for ALI/ARDS treatment, exerts lung-protective effects via multiple pathways. Studies indicate that SIV reduces oxidative stress and inflammation in ALI by inhibiting the JNK/NF-κB pathway and activating the Nrf2/HO-1 pathway, thereby mitigating TNF-α-induced endothelial damage and bacterial-induced lung injury.91 Oxidative stress plays a key role in ALI progression, making antioxidant therapies such as phosphodiesterase (PDE) inhibitors (eg, sildenafil, milrinone) and vitamin C important therapeutic strategies. PDE inhibitors enhance pulmonary microvascular permeability,92 while vitamin C scavenges ROS and improves endothelial integrity.93 Vitamin D has also been shown to modulate inflammation and maintain lung epithelial barrier function, potentially reducing ALI severity.94 β2-adrenergic receptor agonists (eg, salbutamol, terbutaline) help clear alveolar fluid and reduce pulmonary edema; however, their clinical application is limited due to potential adverse effects.95
Additionally, some anesthetics exhibit significant organ-protective effects.96 Advances in drug delivery technologies, such as exosome application, have further improved treatment precision and efficacy.97 Surgical interventions, including ischemic and remote ischemic preconditioning, effectively reduce lung injury by enhancing organ tolerance to ischemia and reducing oxidative stress and inflammatory responses.98 Mesenchymal stem cell therapy shows potential in protecting lung tissue through multiple pathways, such as repairing the intestinal barrier and secreting anti-inflammatory factors.99 Traditional Chinese medicine provides relief for II/R-ALI through multi-target and multi-pathway actions.100
However, the therapeutic effects of these conventional therapy approaches are restricted due to their high toxicity, low drug solubility, and adverse clinical application side effects. Consequently, scientists have recently begun exploring novel nanotherapy methods, aiming to provide more effective treatments by improving drug targeting and reducing toxicity.
Characteristics of Nanomedicine in the Treatment of Acute Lung Injury
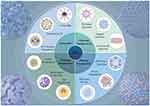
Nanomedicines represent sophisticated drug delivery systems that consist of drugs integrated with carrier materials, which range in size from 1 to 100 nanometers. These carriers encompass liposomes, polymer particles, inorganic nanocarriers, and nanoenzymes (Table 1) (Figure 2).101 These nanomedicines offer advantages such as enhanced drug stability, reduced side effects, targeted delivery, and precise release, with functionalities designed according to specific needs.11 Nanomedicines encapsulate free drugs to prevent their degradation in the bloodstream, thus improving their pharmacokinetic and pharmacodynamic properties. Additionally, modifying the surface of nanomedicines with targeting ligands enhances targeting capability while reducing toxicity and side effects.102 Nanomedicines significantly improve the solubility, stability, and bioactivity of hydrophobic drugs.103 In clinical studies, nanocarriers significantly alter drug biodistribution, increasing drug concentration in inflamed lung areas and enhancing therapeutic efficacy.104 Hence, nanomedicines have extensive potential for application in the treatment of ALI.
 |
Table 1 Therapeutic Effects of Various Nanomaterials on Acute Lung Injury in Animal Models |
Nanomedicines overcome traditional treatment limitations by enhancing therapeutic effects and reducing toxicity.123 Since ALI patients often exhibit multi-organ dysfunction, non-targeted side effects of drugs may exacerbate disease progression. Therefore, targeted drug delivery is of significant clinical importance. Nanocarriers improve the therapeutic efficiency of drugs such as glucocorticoids and PDE4 inhibitors while reducing side effects.124 Additionally, nanocarriers achieve combined delivery of diagnostic and therapeutic agents, enhancing drug delivery efficiency, reducing off-target side effects, and exerting synergistic therapeutic effects.125 Nanoplatforms effectively control acute inflammation and enable comprehensive diagnosis of damaged lungs, demonstrating great potential.126 Despite many advantages of ALI treatment, nanomedicines still face challenges and limitations.127 Some nanomaterials may activate inflammatory pathways, exacerbating inflammation.128 Additionally, after systemic administration, only a small fraction of nanoparticles reach the target site, indicating the need to optimize targeting strategies to improve drug delivery efficiency.129
Applications of Nanomedicine in Acute Lung Injury
Liposome-Based Nanoparticles
Liposomes are formed by amphiphilic components in water, creating closed spherical vesicles with a bilayer structure.130 They can encapsulate both hydrophilic and hydrophobic molecules, meeting various application needs. Liposomes can be prepared by mechanical stirring or ultrasound treatment, with their stability and in vivo half-life enhanced by cholesterol and polyethylene glycol modifications.131 Modified liposomes can avoid interactions with serum proteins, significantly enhancing drug stability.132 Liposomes can also be modified with antibodies or other targeting ligands for active targeting, and can be loaded with various nanoparticles, expanding their applications.130,133 In treating ALI, liposome nanoparticles exhibit significant advantages. Liposomes can encapsulate anti-inflammatory drugs or antioxidants, directly targeting damaged lung areas and reducing systemic side effects.
Raviv et al105 found that liposomes efficiently encapsulate methylprednisolone and N-acetylcysteine, accumulate in inflamed lungs, and reduce inflammation. In vitro experiments showed that liposomes reduced TNFα and NO secretion in LPS-stimulated RAW 264.7 macrophages. In vivo experiments with combined intravenous and endotracheal administration showed that liposomes significantly reduced the secretion of TNFα, IL-6, and IL-1β in bronchoalveolar lavage fluid, which was superior to that of free drug. Excessive activation and uncontrolled infiltration of neutrophils trigger an intense inflammatory response, which is a key mechanism of sepsis-induced ALI. Huang et al106 created a neutrophil membrane-coated liposome loaded with acidic fibroblast growth factor (aFGF@NMLs). In vitro, aFGF@NMLs bind to pro-inflammatory cytokines, promote cellular uptake, significantly reduce inflammatory responses, and boost antioxidant capacity. In vivo, intravenous injection of aFGF@NMLs resulted in nanoparticle accumulation in the damaged lung tissue of ALI mice, reducing pro-inflammatory cytokine secretion, inhibiting lung cell apoptosis, and promoting lung function recovery.
Jiang et al107 designed an l-arginine-modified curcumin liposome (Arg-Cur-Lip) for aerosol inhalation, targeting M1 macrophages to enhance curcumin’s anti-inflammatory effect. In vitro experiments showed that Arg-Cur-Lip inhibited the inflammatory response of LPS-induced RAW264.7 cells, most effectively inhibiting NO, IL-1β, and IL-6 expression. In the LPS-induced rat ALI model, Arg-Cur-Lip significantly reduced TNF-α and IL-6 expression, alleviating lung tissue damage. Quantitative lung pathology scoring showed that the Arg-Cur-Lip group had the best effect.
According to the research mentioned above, liposomes may develop into multipurpose drug delivery systems. By optimizing the structure and function of liposomes, efficient drug delivery and targeted therapy can be achieved, significantly improving therapeutic effects and reducing side effects. Liposome nanoparticles, with high biocompatibility, low toxicity, and multifunctionality, show great promise in treating ALI.
Polymer-Based Nanoparticles
Polymer nanoparticles, made from natural or synthetic polymers, typically exhibit biodegradability and biocompatibility.134 Common materials include natural products (chitosan, alginate, and cyclodextrin) and synthetic polymers (PLGA, PEI, and polyacrylate).135 Synthetic polymers are often coated with polyethylene glycol to reduce toxicity and enhance solubility. Polymer nanoparticles possess high drug-loading capacity, ease of synthesis and characterization, and good biocompatibility and biodegradability, making them ideal carriers for in vivo therapy.136,137
Su et al108 designed a D-mannose-modified polyethyleneimine-block-polycaprolactone micelle (PCL-PEI-mannose) as a dexamethasone-targeted delivery system, demonstrating significant anti-inflammatory effects, demonstrating significant anti-inflammatory effects. Compared to unmodified micelles, mannose-modified micelles had higher cellular uptake and inhibited inflammatory factors in activated RAW264.7 cells. In vivo studies showed that drug-loaded targeted micelles significantly increased lung targeting, reduced the wet/dry ratio of damaged lung tissue, alleviated lung inflammation, and decreased inflammatory cell infiltration, myeloperoxidase activity, and levels of inflammatory mediators in bronchoalveolar lavage fluid. Wan et al109 used mannitol-modified polyethyleneimine (mPEI) to construct an mPEI/pGPX4 gene therapy system. This inhaled system significantly upregulated GPX4 gene expression, inhibited ferroptosis and inflammatory responses, and alleviated ALI in vitro and in vivo. mPEI/pGPX4 nanoparticles significantly reduced lipid peroxidation and ROS levels, improved mitochondrial morphology, and upregulated SLC7A11 and GPX4 expression in LPS-induced BEAS-2B cells. In the LPS-induced mouse model of ALI, mPEI/pGPX4 nanoparticles significantly alleviated lung inflammation and injury, reduced levels of IL-6, IL-1β, and TNF-α, upregulated GPX4 and SLC7A11 expression, decreased ROS levels, and improved mitochondrial morphology.
Solé-Porta et al110 used PLGA nanocapsules as a therapeutic carrier for ARDS. Studies have shown that PLGA NCs can be effectively nebulized into an inhalable aerosol and uniformly distributed in vivo to all regions of the lungs. In vivo studies found that these nanocapsules were internalized by alveolar type II cells and were less likely to be cleared by macrophages. Jin et al111 developed a PLGA-based bionic anti-inflammatory nanoparticle system (PM@Cur-RV NPs) for the treatment of ALI. The system combined PLGA nanoparticles and platelet membrane vesicles (PM) for targeted delivery of curcumin (Cur) and resveratrol (RV). The results showed that PM@Cur-RV NPs significantly reduced pulmonary vascular permeability, attenuated pulmonary edema, decreased the levels of pro-inflammatory cytokines (eg, TNFα, IL-6), and reduced inflammatory cell infiltration in LPS-induced ALI mice. Compared with free Cur and RV, PM@Cur-RV NPs exhibited significantly enhanced anti-inflammatory effects and promoted the polarization of lung macrophages from M1 to M2 and enhanced anti-inflammatory responses in ALI mice.
Ding et al112 evaluated the efficacy of chitosan nanoparticles loaded with methylprednisolone sodium succinate (RBC-MPSS-CSNPs) in a rat model of ALI. The RBC-MPSS-CSNPs significantly reduced pulmonary vascular permeability and pulmonary edema, and significantly decreased the levels of TNF-α and IL-6 in LPS-induced ALI rats. In addition, myeloperoxidase activity and polymorphonuclear leukocyte counts were significantly lower in the BALF of the RBC-MPSS-CSNPs group than those of the control group, and H&E staining showed significant reductions in alveolar hemorrhage and inflammatory cell infiltration. The RBC-MPSS-CSNPs group significantly reduced the lung wet weight/dry weight ratio and total protein concentration in BALF, suggesting that it was effective in reducing pulmonary edema and improving lung injury.
Gao et al113 prepared fifth-generation (G5) poly(amidoamine) (PAMAM) dendrimer (G5.NH2-PBA) nanocarriers functionalized with phenylboronic acid (PBA) for loading fibronectin (FN) and explored their delivery effects and anti-inflammatory effects in macrophages. It was demonstrated that the G5.NH2-PBA/FN (Den/FN) complex significantly reduced LPS-induced secretion of pro-inflammatory cytokines TNF-α and IL-1β, enhanced M2 polarization and anti-inflammatory responses in macrophages, decreased ROS levels, and alleviated inflammation by inhibiting the NF-κB signaling pathway. In vivo experiments in a mouse ALI model showed that Den/FN complex treatment significantly reduced lung inflammation and oxidative stress, improved lung histopathological changes, decreased lung wet weight/dry weight ratio and MPO, TNF-α, IL-1β, and IL-6 levels, and demonstrated good biocompatibility.
Studies have shown that the design and modification of polymer nanoparticles are important in ALI therapy. Specific surface modifications such as mannose or mannitol can improve the targeting of nanoparticles and increase their accumulation in damaged lung tissue. Nanoparticle drug-loading systems enable the controlled release and stable delivery of drugs to improve their bioavailability. Multi-functional polymeric nanoparticles can be loaded with multiple drugs or genes at the same time, enabling combination therapy and enhanced efficacy. Future research should optimize physicochemical properties and biological performance to improve therapeutic efficacy and safety, and strengthen preclinical and clinical studies to promote clinical application in ALI treatment.
Inorganic Nanoparticles
Inorganic nanomaterials have shown great potential in treating ALI. By combining physicochemical properties with biological strategies, these materials enhance targeting and therapeutic effects. Common inorganic nanoparticles include metals and metal oxides, which, through surface modifications and drug conjugation, achieve controlled release and stable delivery, enhancing drug bioavailability. However, their biocompatibility and potential toxicity still require attention.
Wang et al114 found that peptide-coated gold nanoparticles (GNPs) P12 could effectively alleviate LPS-induced ALI. P12 alleviates lung inflammation by reducing inflammatory cell infiltration and raising the anti-inflammatory cytokines, promoting macrophage polarization to the M2 phenotype. It significantly reduced levels of M1 phenotype-associated cytokines (IL-12p40, IFN-γ) and enhanced the production of M2-associated factors (IL-10). P12 is primarily absorbed by lung macrophages, inhibiting the TLR signaling cascade, and promoting M2 macrophage polarization by increasing G-CSF and IL-13, thereby mitigating pulmonary inflammatory responses. Haupenthal et al115 found that intraperitoneally administered gold nanoparticles (GNPs) had strong anti-inflammatory and antioxidant effects in the ALI model. GNP treatment greatly decreased LPS-induced pro-inflammatory cytokines IFN-γ and IL-6 decreased oxidant production (nitrites and DCFH), and reduced oxidative damage (carbonyl and thiol groups). Additionally, GNP treatment significantly reduced superoxide dismutase and catalase activity. Histological analysis showed that GNP treatment could reverse LPS-induced increases in alveolar septal thickness.
In an LPS-induced ALI mouse model, porous Se@SiO2 nanoparticles demonstrated significant antioxidant and anti-inflammatory effects. Wang et al116 found that Se@SiO2 nanoparticle treatment greatly lowered levels of pro-inflammatory cytokines IL-1β, CCL-2, and IL-6 induced by LPS, decreased oxidative stress and mitochondrial ROS production, and reduced alveolar-capillary barrier permeability. Additionally, Se@SiO2 nanoparticle treatment improved mitochondrial function, increased expression of tight junction proteins ZO-1 and E-Ca, reduced pulmonary histopathological damage, and protected airway epithelial cells from LPS-induced oxidative damage and inflammatory responses. García-Fernández et al117 developed a nanodevice using mesoporous silicon nanoparticles (MSNs) loaded with dexamethasone and capped with a peptide targeting the TNFR1 receptor (TNFR-Dex-MSN). M1 macrophages overexpressing the TNFR1 receptor selectively internalize this nanodevice, which releases dexamethasone upon enzymatic hydrolysis of the capping peptide. This reduces levels of TNF-α and IL-1β. TNFR-Dex-MSNs accumulated in injured lungs, significantly reducing inflammatory responses and lung injury while minimizing dexamethasone side effects.
Li et al118 developed a dry powder inhaler based on γ-cyclodextrin metal-organic frameworks (CD-MOFs) for targeted delivery of paeonol (PAE) to the lungs for treating ALI. PAE-loaded CD-MOF particles exhibited rapid release characteristics in animal experiments, significantly improving PAE absorption and bioavailability. In a rat model, the PAE-CD-MOF significantly increased PAE absorption in lung tissue. Histopathological testing and decreased levels of inflammatory markers showed its therapeutic efficacy. Wang et al119 developed nanoparticles based on zeolitic imidazolate framework-8 (ZIF-8) to encapsulate the anticancer drug plumbagin (PLB) as a drug delivery system for treating ALI. ZIF-8 nanoparticles uniformly encapsulated PLB through physical adsorption. In animal models, PLB@ZIF-8 dramatically reduced collagen fiber deposition after severe lung damage, lowered inflammatory factors TGF-β and IL-6, and suppressed expression of collagen I, α-SMA, and TNF-α. Compared to free PLB, PLB@ZIF-8 exhibited better anti-inflammatory effects, suggesting that ZIF-8-encapsulated PLB could become a new therapy for treating severe lung injury.
Inorganic nanoparticles have shown great potential in treating ALI. By combining physicochemical properties with biological strategies, these materials can achieve targeted delivery and controlled release of drugs, significantly enhancing drug bioavailability. These studies indicate that inorganic nanoparticles can effectively alleviate ALI symptoms by reducing pro-inflammatory cytokine levels, decreasing oxidative stress, and improving histopathological outcomes. However, the biocompatibility and potential toxicity of inorganic nanomaterials still require further research to ensure their safety and efficacy in clinical applications.
Nanozymes
Nanozymes, as substitutes for natural enzymes, have become a research hotspot due to their enhanced biocatalytic performance, adjustable activity, and diverse functions. The introduction of nanozymology has challenged the traditional concept of the biological inertness of inorganic materials, sparking interest in the characteristics and multifunctionality of nanozymes.138 Although research on nanozymes in respiratory diseases is limited, they show potential in bioanalysis, biomedical imaging, and therapy.139 Through rational design, nanozymes can significantly enhance enzyme activity, achieve diversified applications, and possess antibacterial properties that do not easily lead to resistance.140 Some nanozymes exhibit multiple activities, form cascade reactions, improve reaction efficiency, and produce synergistic effects in fields such as antioxidation and anticancer.141 Overall, nanozymes combine the catalytic functions of enzymes with the unique physicochemical properties of nanomaterials, overcoming the limitations of traditional enzyme sensors and showing broad application prospects in respiratory diseases.
In ALI, sepsis-induced lung inflammation and excessive inflammatory cell infiltration are the main pathological features. Studies have shown that impaired phagocytosis can exacerbate this condition.142 Ji et al120 designed an antioxidative nanozyme (AOzyme) coated with apoptotic cell membranes (ACM), forming an inhalable phagocytosis-promoting nanozyme (AOzyme@ACM). AOzyme@ACM improves macrophage perception of “eat me” signals by imitating apoptotic bodies, while also clearing excess intracellular ROS, significantly increasing apoptotic cell clearance. AOzyme@ACM significantly inhibits inflammatory responses, promotes macrophage polarization to the M2 phenotype, and alleviates ALI in endotoxemic mice. In vivo experiments demonstrated that mice treated with AOzyme@ACM had excellent apoptotic cell clearance, controlled inflammatory cytokine production, reduced inflammatory cell infiltration, and lowered pulmonary edema.
In the ALI model constructed by Yuan et al,121 iron-curcumin-based nanoparticles (Fe-Cur NPs) demonstrated significant anti-inflammatory and ROS scavenging abilities. Fe-Cur NPs decreased inflammation by downregulating cytokines (eg, TNF-α and IL-6) and blocking NLRP3 and NF-κB signaling pathways. Experiments showed that both intratracheal and intravenous injections of Fe-Cur NPs exhibited good therapeutic effects, including reducing inflammatory cytokines, decreasing inflammatory cell infiltration and pulmonary edema, and effectively scavenging ROS in lung tissues. Additionally, Fe-Cur NPs reduced macrophage and T cell levels, inhibiting the cytokine storm induced by ALI.
Lui et al122 found that carbon dot (C-dot) superoxide dismutase (SOD) nanozymes exhibited significant therapeutic effects in ALI. Studies have shown that C-dot SOD nanozymes achieve in vivo bioimaging and ROS scavenging through their high SOD-like activity and red fluorescence. It was shown that C-dot SOD achieve in vivo bioimaging and ROS scavenging through their high SOD-like activity and red fluorescence. In vivo experiments showed that C-dot SOD nano-enzymes aggregated in injured lung tissues and effectively protected mice from oxidative damage by scavenging ROS and decreasing pro-inflammatory factor levels. Compared with the clinical drug dexamethasone, C-dot SOD showed comparable therapeutic effects. In addition, C-dot SOD effectively enter cells and accumulate in mitochondria, protecting cells from oxidative damage by scavenging ROS.
Nanoenzymes, as natural enzyme substitutes, have shown great potential in the treatment of ALI due to their enhanced biocatalytic properties and versatility. Compared with conventional drugs, nanoenzymes offer unique advantages in reducing pro-inflammatory factors, enhancing anti-inflammatory factors and protecting cells from oxidative damage. These studies provide a solid foundation for the application of nanoenzymes in ALI, but their biosafety and clinical translation still need to be further explored and validated.
Exosomes
Exosomes are nanoscale membrane-bound vesicles secreted by a variety of cell types, including immune cells, tumor cells, and stem cells. They typically range in diameter from 30 to 150 nm and are characterized by a classical lipid bilayer structure.143 Exosome biogenesis primarily occurs through the endosomal pathway: invagination of the plasma membrane leads to the formation of early endosomes, which subsequently mature into late endosomes and develop into multivesicular bodies (MVBs) containing numerous intraluminal vesicles.144 While some MVBs fuse with lysosomes for degradation, others merge with the plasma membrane, releasing their internal vesicles into the extracellular space as exosomes via exocytosis.145 Exosomes are abundantly present in various bodily fluids, including blood, urine, saliva, and breast milk.146 They express a range of characteristic surface biomarkers, such as CD63, CD9, CD81, Tsg101, Alix, and heat shock proteins.147 As key mediators of intercellular communication, exosomes can carry a diverse array of bioactive molecules, including proteins, lipids, messenger RNAs, microRNAs, and other non-coding RNAs.148 These cargo molecules are selectively loaded into exosomes and can be transferred to recipient cells through multiple mechanisms: direct fusion with the target cell membrane to release their contents, internalization via endocytosis, or interaction between exosomal membrane ligands and cell surface receptors to activate downstream signaling pathways.149 Exosomes, as key intercellular communicators, exhibit multiple biological effects in the prevention and treatment of ALI, including anti-inflammatory, anti-apoptotic, and promotion of lung tissue regeneration.
Excessive activation of the inflammatory response is one of the key pathological features of ALI, manifested by massive recruitment of neutrophils and macrophages, as well as significantly elevated levels of proinflammatory factors (such as TNF-α, IL-1β, and MIP-2).150 Research shows that exosomes derived from mesenchymal stem cells (MSCs) play an important role in the anti-inflammatory process. For example, ischemia-pretreated MSC-exosomes can effectively reduce neutrophil infiltration in the lungs and downregulate MIP-2 expression in an LPS-induced ALI mouse model.151 In vitro studies have also shown that MSC-exosomes can inhibit the release of various pro-inflammatory factors and promote the expression of anti-inflammatory factors TGF-β, thereby inhibiting Th17 cell differentiation and inducing the generation of regulatory T cells.152 In various ALI models, such as phosgene, LPS, and sepsis, MSC-exosomes have demonstrated the ability to regulate the balance of inflammatory factors by inhibiting pro-inflammatory factors such as TNF-α and IL-6 and enhancing the expression of IL-10, thereby exerting a significant immunoregulatory effect.153–156 In addition, it can induce macrophage polarization toward the M2 phenotype by activating the Stat6 and MafB signaling pathways and upregulating the expression of TSG-6 and IL-10, further enhancing its anti-inflammatory potential.157
The apoptosis of alveolar epithelial cells and endothelial cells plays a significant role in the pathogenesis of ALI.158 Multiple studies have shown that MSC-derived exosomes can effectively downregulate the expression of PTEN and PDCD4 by carrying functional microRNAs such as miR-21-5p, thereby inhibiting endogenous and exogenous apoptosis pathways, significantly reducing the activity levels of caspase-3/-8/-9, and alleviating lung injury caused by ischemia/reperfusion.159 In addition, exosomes can inhibit LPS- or bleomycin-induced apoptosis of alveolar epithelial cells through multiple mechanisms such as negatively regulating NLRP3 inflammasome activity, alleviating mitochondrial DNA damage, and activating the SIRT1 pathway.160 Exosomes derived from adipose-derived stem cells also exhibit significant cell protective effects, alleviating sepsis-related lung injury by inducing autophagy, reducing proinflammatory factor levels, and lowering apoptosis rates.161
A prominent feature of ALI is impaired alveolar-capillary barrier function, and effective repair of epithelial and endothelial cell function is critical for lung tissue regeneration and functional recovery.158 Studies have confirmed that MSC-exosomes can enhance the proliferation and migration ability of endothelial cells and promote angiogenesis by activating the AKT/mTOR and HIF-1α/VEGF signaling pathways, thereby accelerating the tissue repair process.162,163 In addition, exosomes derived from endothelial progenitor cells also have a repair effect, which can improve the structure of alveolar epithelial cells and enhance the expression of tight junction proteins, thereby strengthening the barrier function and reducing lung permeability.164 Exosomes derived from type II alveolar epithelial cells have also been found to enhance their own survival and regenerative abilities, promoting the re-epithelialization process in damaged areas.165
Exosomes, as crucial mediators of intercellular communication, possess excellent biocompatibility, structural stability, and the ability to deliver a variety of bioactive molecules in a targeted manner. They have demonstrated extensive and profound therapeutic potential in the prevention and treatment of ALI. Through multiple mechanisms, exosomes can coordinate the regulation of inflammatory responses, inhibit cell apoptosis, promote tissue repair, and restore the integrity of the alveolar-capillary barrier. These effects provide a solid theoretical foundation and experimental support for the development of stem cell-derived exosome therapies for ALI, while also offering important directions for their future clinical translation.
Conclusion and Future Prospects
II/R injury not only severely damages the intestinal tract but also triggers ALI through mechanisms such as inflammation and oxidative stress. During II/R, the destruction of the intestinal mucosal barrier leads to the translocation of bacteria and endotoxins into the circulation, thereby triggering SIRS and multi-organ failure. ALI, as a common and serious complication of II/R, involves a complex pathogenesis including oxidative stress, inflammatory responses, apoptosis, ferroptosis, and pyroptosis. Although conventional therapies can alleviate ALI symptoms, their efficacy remains limited, and side effects are a concern. Therefore, there is an urgent need for novel therapeutic strategies.
Nanomedicine has emerged as a promising approach for treating II/R-ALI. Leveraging unique physicochemical properties, nanomedicines improve drug targeting and bioavailability while reducing systemic side effects. Precise delivery of drugs via nanocarriers enhances therapeutic effects at the sites of inflammation and tissue injury. Recent advances in nanomedicine research for ALI treatment have demonstrated significant progress and promising clinical potential.
However, challenges remain regarding the long-term toxicity and biocompatibility of nanoparticles, which are not yet fully understood. Ensuring the safety of nanomaterials requires comprehensive toxicological studies. Additionally, the complex and costly manufacturing processes of nanomaterials present technical and economic barriers to large-scale production. The lack of standardized regulations and guidelines for nanomedicine development, testing, and approval may result in variable product quality, hindering broader clinical application. Furthermore, the biodistribution and metabolism of nanoparticles in vivo are complex, and strategies to control and predict their behavior need further investigation.
Future Prospects
Despite these challenges, the future of nanomedicine in ALI treatment remains bright. Future research is expected to focus on developing multifunctional nanoplatforms that integrate diagnostic and therapeutic capabilities. Such platforms will combine imaging, targeted drug delivery, and therapy to improve treatment efficacy and minimize adverse effects. Personalized nanomedicine, tailored to the patient’s genetic profile, disease state, and physiological conditions, holds promise for enhancing treatment outcomes.
Moreover, the design of smart nanosystems capable of responding to specific internal stimuli—such as pH, temperature, or enzymes—will enable controlled drug release under defined conditions, further increasing therapeutic precision. The advancement of nanomedicine relies heavily on interdisciplinary collaboration among materials science, chemistry, biology, medicine, and engineering, which will accelerate technological innovation and clinical translation.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li G, Wang S, Fan Z. Oxidative stress in intestinal ischemia-reperfusion. Front Med. 2022;8:750731. doi:10.3389/fmed.2021.750731
2. Ge P, Luo Y, Okoye CS, et al. Intestinal barrier damage, systemic inflammatory response syndrome, and acute lung injury: a troublesome trio for acute pancreatitis. Biomed Pharmacother. 2020;132:110770. doi:10.1016/j.biopha.2020.110770
3. Bagnacci G, Guerrini S, Gentili F, et al. Non-occlusive mesenteric ischemia (NOMI) and prognostic signs at CT: reperfusion or not reperfusion that is the question! Abdom Radiol. 2022;47(5):1603–1613. doi:10.1007/s00261-021-03317-z
4. Liao S, Luo J, Kadier T, Ding K, Chen R, Meng Q. Mitochondrial DNA release contributes to intestinal ischemia/reperfusion injury. Front Pharmacol. 2022;13:854994. doi:10.3389/fphar.2022.854994
5. Zhan Y, Ling Y, Deng Q, et al. HMGB1-mediated neutrophil extracellular trap formation exacerbates intestinal ischemia/reperfusion-induced acute lung injury. J Immunol. 2022;208(4):968–978. doi:10.4049/jimmunol.2100593
6. Li G, Zhang Y, Fan Z. Cellular signal transduction pathways involved in acute lung injury induced by intestinal ischemia‐reperfusion. Oxid Med Cell Longev. 2021;2021(1):9985701. doi:10.1155/2021/9985701
7. Mowery NT, Terzian WH, Nelson AC. Acute lung injury. Curr Problems Surg. 2020;57(5):100777. doi:10.1016/j.cpsurg.2020.100777
8. Long ME, Mallampalli RK, Horowitz JC. Pathogenesis of pneumonia and acute lung injury. Clin Sci. 2022;136(10):747–769. doi:10.1042/CS20210879
9. Mokra D. Acute lung injury–from pathophysiology to treatment. Physiological Res. 2020;69(Suppl 3):S353. doi:10.33549/physiolres.934602
10. Chen K, Wu J, Yarin A. Electrospun membranes filtering 100 nm particles from air flow by means of the van der Waals and Coulomb forces. J Membr Sci. 2022;644:120138. doi:10.1016/j.memsci.2021.120138
11. Raj S, Khurana S, Choudhari R, et al. Specific targeting cancer cells with nanoparticles and drug delivery in cancer therapy. Paper presented at: Seminars in cancer biology. 2021.
12. Tian H, Zhang T, Qin S, et al. Enhancing the therapeutic efficacy of nanoparticles for cancer treatment using versatile targeted strategies. J Hematol Oncol. 2022;15(1):132.
13. Wang K, Rong G, Gao Y, et al. Fluorous‐tagged peptide nanoparticles ameliorate acute lung injury via lysosomal stabilization and inflammation inhibition in pulmonary macrophages. Small. 2022;18(40):2203432. doi:10.1002/smll.202203432
14. Sultana A, Zare M, Thomas V, Kumar TS, Ramakrishna S. Nano-based drug delivery systems: conventional drug delivery routes, recent developments and future prospects. Med Drug Discovery. 2022;15:100134. doi:10.1016/j.medidd.2022.100134
15. Metselaar JM, Lammers T. Challenges in nanomedicine clinical translation. Drug Delivery Transl Res. 2020;10(3):721–725. doi:10.1007/s13346-020-00740-5
16. Liu X, Tang I, Wainberg ZA, Meng H. Safety considerations of cancer nanomedicine—a key step toward translation. Small. 2020;16(36):2000673. doi:10.1002/smll.202000673
17. Woźniak D, Cichy W, Przysławski J, Drzymała-Czyż S. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Adv Med Sci. 2021;66(2):284–292. doi:10.1016/j.advms.2021.05.003
18. Deng F, Lin Z-B, Sun Q-S, et al. The role of intestinal microbiota and its metabolites in intestinal and extraintestinal organ injury induced by intestinal ischemia reperfusion injury. Int J Bio Sci. 2022;18(10):3981. doi:10.7150/ijbs.71491
19. Abd El-Baset SA, Abd El-haleem MR, Abdul-Maksoud RS, Kattaia AA. Mesna ameliorates acute lung injury induced by intestinal ischemia–reperfusion in rats. Sci Rep. 2021;11(1):13356. doi:10.1038/s41598-021-92653-7
20. Kligerman S. Pathogenesis, imaging, and evolution of acute lung injury. Radiologic Clinics. 2022;60(6):925–939. doi:10.1016/j.rcl.2022.06.005
21. Meyer NJ, Gattinoni L, Calfee CS. Acute respiratory distress syndrome. Lancet. 2021;398(10300):622–637.
22. Zhongyin Z, Wei W, Juan X, Guohua F. Isoliquiritin apioside relieves intestinal ischemia/reperfusion-induced acute lung injury by blocking Hif-1α-mediated ferroptosis. Int Immunopharmacol. 2022;108:108852. doi:10.1016/j.intimp.2022.108852
23. Li Y, Cao Y, Xiao J, et al. Inhibitor of apoptosis-stimulating protein of p53 inhibits ferroptosis and alleviates intestinal ischemia/reperfusion-induced acute lung injury. Cell Death Differ. 2020;27(9):2635–2650. doi:10.1038/s41418-020-0528-x
24. Liu N, Xu H, Sun Q, et al. The role of oxidative stress in hyperuricemia and xanthine oxidoreductase (XOR) inhibitors. Oxid Med Cell Longev. 2021;2021(1):1470380. doi:10.1155/2021/1470380
25. Martín Giménez VM, de Las Heras N, Ferder L, Lahera V, Reiter RJ, Manucha W. Potential effects of melatonin and micronutrients on mitochondrial dysfunction during a cytokine storm typical of oxidative/inflammatory diseases. Diseases. 2021;9(2):30.
26. Engwa GA, Nweke FN, Nkeh-Chungag BN. Free radicals, oxidative stress-related diseases and antioxidant supplementation. Alternative Ther Health Med. 2022;28(1).
27. Unsal V, Cicek M, Sabancilar İ. Toxicity of carbon tetrachloride, free radicals and role of antioxidants. Rev Environm Health. 2021;36(2):279–295. doi:10.1515/reveh-2020-0048
28. Bhol NK, Bhanjadeo MM, Singh AK, et al. The interplay between cytokines, inflammation, and antioxidants: mechanistic insights and therapeutic potentials of various antioxidants and anti-cytokine compounds. Biomed Pharmacother. 2024;178:117177.
29. Huang Y, Xu W, Zhou R. NLRP3 inflammasome activation and cell death. Cell Mol Immunol. 2021;18(9):2114–2127. doi:10.1038/s41423-021-00740-6
30. Wang B, Wang Y, Zhang J, et al. ROS-induced lipid peroxidation modulates cell death outcome: mechanisms behind apoptosis, autophagy, and ferroptosis. Arch Toxicol. 2023;97(6):1439–1451. doi:10.1007/s00204-023-03476-6
31. Keskinidou C, Vassiliou AG, Dimopoulou I, Kotanidou A, Orfanos SE. Mechanistic understanding of lung inflammation: recent advances and emerging techniques. J Inflamm Res. 2022;15:3501–3546. doi:10.2147/JIR.S282695
32. Li D, Cong Z, Yang C, Zhu X. Inhibition of LPS-induced Nox2 activation by VAS2870 protects alveolar epithelial cells through eliminating ROS and restoring tight junctions. Biochem Biophys Res Commun. 2020;524(3):575–581. doi:10.1016/j.bbrc.2020.01.134
33. Bormann T, Maus R, Stolper J, et al. Role of matrix metalloprotease-2 and MMP-9 in experimental lung fibrosis in mice. Respir Res. 2022;23(1):180. doi:10.1186/s12931-022-02105-7
34. Tong Y, Yu Z, Chen Z, et al. The HIV protease inhibitor Saquinavir attenuates sepsis-induced acute lung injury and promotes M2 macrophage polarization via targeting matrix metalloproteinase-9. Cell Death Dis. 2021;12(1):67. doi:10.1038/s41419-020-03320-0
35. Pan L, Cheng Y, Yang W, et al. Nintedanib ameliorates bleomycin-induced pulmonary fibrosis, inflammation, apoptosis, and oxidative stress by modulating PI3K/Akt/mTOR pathway in mice. Inflammation. 2023;46(4):1531–1542. doi:10.1007/s10753-023-01825-2
36. Zhang Q, Wang Z, Zhu J, Peng Z, Tang C. Ferulic acid regulates miR-17/PTEN axis to inhibit LPS-induced pulmonary microvascular endothelial cells apoptosis through activation of PI3K/Akt pathway. J Toxicol Sci. 2022;47(2):61–69. doi:10.2131/jts.47.61
37. Denning N-L, Aziz M, Ochani M, Prince JM, Wang P. Inhibition of a triggering receptor expressed on myeloid cells-1 (TREM-1) with an extracellular cold-inducible RNA-binding protein (eCIRP)-derived peptide protects mice from intestinal ischemia-reperfusion injury. Surgery. 2020;168(3):478–485. doi:10.1016/j.surg.2020.04.010
38. Liu C, Chu D, Kalantar‐Zadeh K, George J, Young HA, Liu G. Cytokines: from clinical significance to quantification. Adv Sci. 2021;8(15):2004433. doi:10.1002/advs.202004433
39. Megha K, Joseph X, Akhil V, Mohanan P. Cascade of immune mechanism and consequences of inflammatory disorders. Phytomedicine. 2021;91:153712. doi:10.1016/j.phymed.2021.153712
40. Goyert SM. Gene knockout technology and the host response to endotoxin: role of CD14 and other inflammatory mediators. In: Endotoxin in Health and Disease. CRC Press; 2020:781–793.
41. Chen Y, Pu W, Maswikiti EP, et al. Intestinal congestion and reperfusion injury: damage caused to the intestinal tract and distal organs. Biosci Rep. 2021;41(9):BSR20211560. doi:10.1042/BSR20211560
42. Wang Y, Zhu C-L, Li P, et al. The role of G protein-coupled receptor in neutrophil dysfunction during sepsis-induced acute respiratory distress syndrome. Front Immunol. 2023;14:1112196. doi:10.3389/fimmu.2023.1112196
43. Liu X, Yang B, Tan Y-F, et al. The role of AMPK-Sirt1-autophagy pathway in the intestinal protection process by propofol against regional ischemia/reperfusion injury in rats. Int Immunopharmacol. 2022;111:109114. doi:10.1016/j.intimp.2022.109114
44. Di Vincenzo F, Del Gaudio A, Petito V, Lopetuso LR, Scaldaferri F. Gut microbiota, intestinal permeability, and systemic inflammation: a narrative review. Int Emerg Med. 2024;19(2):275–293. doi:10.1007/s11739-023-03374-w
45. Tang J, Xu L, Zeng Y, Gong F. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol. 2021;91:107272. doi:10.1016/j.intimp.2020.107272
46. Zhang Q, Li J, Zhong H, Xu Y. The mechanism of nicotinamide on reducing acute lung injury by inhibiting MAPK and NF-κB signal pathway. Mol Med. 2021;27(1):1–11. doi:10.1186/s10020-021-00376-2
47. Ghafouri-Fard S, Shoorei H, Poornajaf Y, et al. NLRP3: role in ischemia/reperfusion injuries. Front Immunol. 2022;13:926895. doi:10.3389/fimmu.2022.926895
48. Zhang W-J, Li K-Y, Lan Y, Zeng H-Y, Chen S-Q, Wang H. NLRP3 Inflammasome: a key contributor to the inflammation formation. Food Chem Toxicol. 2023;174:113683. doi:10.1016/j.fct.2023.113683
49. Yin H, Fang L, Wang L, et al. Acute silica exposure triggers pulmonary inflammation through macrophage pyroptosis: an experimental simulation. Front Immunol. 2022;13:874459. doi:10.3389/fimmu.2022.874459
50. Gerber A, Goldklang M, Stearns K, et al. Attenuation of pulmonary injury by an inhaled MMP inhibitor in the endotoxin lung injury model. Am J Physiol Lung Cell Mol Physiol. 2020;319(6):L1036–L1047.
51. Liang Y, Yang N, Pan G, Jin B, Wang S, Ji W. Elevated IL-33 promotes expression of MMP2 and MMP9 via activating STAT3 in alveolar macrophages during LPS-induced acute lung injury. Cell Mol Biol Lett. 2018;23(1):1–13. doi:10.1186/s11658-018-0117-x
52. Lemjabbar H, Gosset P, Lechapt-Zalcman E, et al. Overexpression of alveolar macrophage gelatinase B (MMP-9) in patients with idiopathic pulmonary fibrosis: effects of steroid and immunosuppressive treatment. Am J Respir Cell Mol Biol. 1999;20(5):903–913. doi:10.1165/ajrcmb.20.5.3260
53. Corbel M, Boichot E, Lagente V. Role of gelatinases MMP-2 and MMP-9 in tissue remodeling following acute lung injury. Braz J Med Biol Res. 2000;33(7):749–754. doi:10.1590/S0100-879X2000000700004
54. Steenport M, Khan K, Du B, Barnhard SE, Dannenberg AJ, Falcone DJ. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: evidence for the role of TNF-α and cyclooxygenase-2. J Immunol. 2009;183(12):8119–8127. doi:10.4049/jimmunol.0901925
55. Pérez-ramos J, de Lourdes Segura-Valdez M, Vanda B, Selman M, Pardo A. Matrix metalloproteinases 2, 9, and 13, and tissue inhibitors of metalloproteinases 1 and 2 in experimental lung silicosis. Am J Respir Crit Care Med. 1999;160(4):1274–1282. doi:10.1164/ajrccm.160.4.9808006
56. Mithun MH, Kar A, Prome SM, Jahan I, Akter A, Hasan SE. A comprehensive review on cell death. J Knowledge Learn Sci Technol. 2023;2(3):170–188. doi:10.60087/jklst.vol2.n3.p188
57. Subramanian S, Geng H, Tan X-D. Cell death of intestinal epithelial cells in intestinal diseases. Sheng Li Xue Bao. 2020;72(3):308.
58. Patankar JV, Becker C. Cell death in the gut epithelium and implications for chronic inflammation. Nat Rev Gastroenterol Hepatol. 2020;17(9):543–556. doi:10.1038/s41575-020-0326-4
59. Denning TL, Takaishi H, Crowe SE, Boldogh I, Jevnikar A, Ernst PB. Oxidative stress induces the expression of Fas and Fas ligand and apoptosis in murine intestinal epithelial cells. Free Radic Biol Med. 2002;33(12):1641–1650. doi:10.1016/S0891-5849(02)01141-3
60. Han Y-H, Wang Y, Lee S-J, Jin M-H, Sun H-N, Kwon T. Regulation of anoikis by extrinsic death receptor pathways. Cell Commun Signaling. 2023;21(1):227. doi:10.1186/s12964-023-01247-5
61. Ranjan K, Pathak C. Cellular dynamics of fas-associated death domain in the regulation of cancer and inflammation. Int J Mol Sci. 2024;25(6):3228. doi:10.3390/ijms25063228
62. Wang L, Zhang Y, Song Z, Liu Q, Fan D, Song X. Ginsenosides: a potential natural medicine to protect the lungs from lung cancer and inflammatory lung disease. Food Funct. 2023;14(20):9137–9166. doi:10.1039/D3FO02482B
63. Koohpeyma H, Goudarzi I, Elahdadi Salmani M, Lashkarbolouki T, Shabani M. Folic acid protects rat cerebellum against oxidative damage caused by homocysteine: the expression of Bcl-2, Bax, and Caspase-3 apoptotic genes. Neurotox Res. 2020;37(3):564–577. doi:10.1007/s12640-019-00119-6
64. Zhang S, Rao S, Yang M, Ma C, Hong F, Yang S. Role of mitochondrial pathways in cell apoptosis during He-patic ischemia/reperfusion injury. Int J Mol Sci. 2022;23(4):2357. doi:10.3390/ijms23042357
65. Pepper C, Bentley P. The role of the Bcl-2 family in the modulation of apoptosis. Programmed Cell Death Animals Plants. 2021;43–53.
66. Chen Z, Wang Z, Liu D, et al. Critical role of caveolin-1 in intestinal ischemia reperfusion by inhibiting protein kinase C βII. Free Radic Biol Med. 2023;194:62–70. doi:10.1016/j.freeradbiomed.2022.11.030
67. Wang G, Chen Z, Zhang F, et al. Blockade of PKCβ protects against remote organ injury induced by intestinal ischemia and reperfusion via a p66shc-mediated mitochondrial apoptotic pathway. Apoptosis. 2014;19(9):1342–1353. doi:10.1007/s10495-014-1008-x
68. Feng D, Yao J, Wang G, et al. Inhibition of p66Shc-mediated mitochondrial apoptosis via targeting prolyl-isomerase Pin1 attenuates intestinal ischemia/reperfusion injury in rats. Clin Sci. 2017;131(8):759–773. doi:10.1042/CS20160799
69. Wang X, Hua P, He C, Chen M. Non-apoptotic cell death-based cancer therapy: molecular mechanism, pharmacological modulators, and nanomedicine. Acta Pharmaceutica Sinica B. 2022;12(9):3567–3593. doi:10.1016/j.apsb.2022.03.020
70. Li J, Cao F, Yin H-L, et al. Ferroptosis: past, present and future. Cell Death Dis. 2020;11(2):88. doi:10.1038/s41419-020-2298-2
71. Chen X, Li J, Kang R, Klionsky DJ, Tang D. Ferroptosis: machinery and regulation. Autophagy. 2021;17(9):2054–2081. doi:10.1080/15548627.2020.1810918
72. Liu X, Pan B, Wang X, et al. Ischemia/reperfusion-activated ferroptosis in the early stage triggers excessive inflammation to aggregate lung injury in rats. Front Med. 2023;10:1181286. doi:10.3389/fmed.2023.1181286
73. Dong H, Qiang Z, Chai D, et al. Nrf2 inhibits ferroptosis and protects against acute lung injury due to intestinal ischemia reperfusion via regulating SLC7A11 and HO-1. Aging. 2020;12(13):12943. doi:10.18632/aging.103378
74. Fusco R, Siracusa R, Genovese T, Cuzzocrea S, Di Paola R. Focus on the role of NLRP3 inflammasome in diseases. Int J Mol Sci. 2020;21(12):4223. doi:10.3390/ijms21124223
75. Fu J, Wu H. Structural mechanisms of NLRP3 inflammasome assembly and activation. Ann Rev Immunol. 2023;41(1):301–316. doi:10.1146/annurev-immunol-081022-021207
76. Wei T, Zhang C, Song Y. Molecular mechanisms and roles of pyroptosis in acute lung injury. Chinese Med J. 2022;135(20):2417–2426. doi:10.1097/CM9.0000000000002425
77. Li W, Yang K, Li B, et al. Corilagin alleviates intestinal ischemia/reperfusion-induced intestinal and lung injury in mice via inhibiting NLRP3 inflammasome activation and pyroptosis. Front Pharmacol. 2022;13:1060104. doi:10.3389/fphar.2022.1060104
78. Wei Y, Yang L, Pandeya A, Cui J, Zhang Y, Li Z. Pyroptosis-induced inflammation and tissue damage. J Mol Biol. 2022;434(4):167301. doi:10.1016/j.jmb.2021.167301
79. Wang Z, Li Z, Feng D, et al. Autophagy induction ameliorates inflammatory responses in intestinal ischemia–reperfusion through inhibiting NLRP3 inflammasome activation. Shock. 2019;52(3):387–395. doi:10.1097/SHK.0000000000001259
80. El-Malkey NF, Alsemeh AE, Ashour WM, Hassan NH, Edrees HM. Fetuin-A exerts a protective effect against experimentally induced intestinal ischemia/reperfusion by suppressing autophagic cell death. Exp Biol Med. 2021;246(11):1307–1317. doi:10.1177/1535370221995207
81. Jiang M, Wan S, Dai X, et al. Protective effect of ghrelin on intestinal I/R injury in rats. Open Med. 2022;17(1):1308–1317. doi:10.1515/med-2022-0520
82. Wang R, Wang Y, Hu L, Lu Z, Wang X. Inhibition of complement C5a receptor protects lung cells and tissues against lipopolysaccharide-induced injury via blocking pyroptosis. Aging. 2021;13(6):8588. doi:10.18632/aging.202671
83. Zheng J, Li Y, Kong X, Guo J. Exploring immune-related pathogenesis in lung injury: providing new insights Into ALI/ARDS. Biomed Pharmacother. 2024;175:116773. doi:10.1016/j.biopha.2024.116773
84. Mokra D, Mokry J. Oxidative stress in experimental models of acute lung injury. Oxidative Stress Lung Dis. 2020;2:25–57.
85. Lv S, Zhao X, Ma C, et al. Advancements in the study of acute lung injury resulting from intestinal ischemia/reperfusion. Front Med. 2024;11:1399744. doi:10.3389/fmed.2024.1399744
86. Reichardt SD, Amouret A, Muzzi C, et al. The role of glucocorticoids in inflammatory diseases. Cells. 2021;10(11):2921. doi:10.3390/cells10112921
87. Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324(13):1307–1316. doi:10.1001/jama.2020.17021
88. Ren Y, Li L, Wang -M-M, et al. Pravastatin attenuates sepsis-induced acute lung injury through decreasing pulmonary microvascular permeability via inhibition of Cav-1/eNOS pathway. Int Immunopharmacol. 2021;100:108077. doi:10.1016/j.intimp.2021.108077
89. Subir R, Jagat J M, kalyan K G. Pros and cons for use of statins in people with coronavirus disease-19 (COVID-19). Diabetes Metab Syndr. 2020;14(5):1225–1229. doi:10.1016/j.dsx.2020.07.011
90. Marinaccio L, Stefanucci A, Scioli G, et al. Peptide human neutrophil elastase inhibitors from natural sources: an overview. Int J Mol Sci. 2022;23(6):2924. doi:10.3390/ijms23062924
91. Zhang H, Zeng J, Li J, et al. Sivelestat sodium attenuates acute lung injury by inhibiting JNK/NF-κB and activating Nrf2/HO-1 signaling pathways. Biomolecules Biomed. 2023;23(3):457. doi:10.17305/bb.2022.8549
92. Moore HM, Drucker NA, Hosfield BD, Shelley WC, Markel TA. Sildenafil as a rescue agent following intestinal ischemia and reperfusion injury. J Surg Res. 2020;246:512–518. doi:10.1016/j.jss.2019.09.037
93. Fowler III AA, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261. doi:10.1001/jama.2019.11825
94. Ahmad S, Arora S, Khan S, et al. Vitamin D and its therapeutic relevance in pulmonary diseases. J Nutr Biochem. 2021;90:108571. doi:10.1016/j.jnutbio.2020.108571
95. Beng H, Hu J, Wang S, Liang X, Qin H, Tan W. Effects of R-salbutamol on the inflammatory response and acute lung injury in endotoxemic mice. Int Immunopharmacol. 2023;121:110482. doi:10.1016/j.intimp.2023.110482
96. Wang J, Zhang W, Wu G. Intestinal ischemic reperfusion injury: recommended rats model and comprehensive review for protective strategies. Biomed Pharmacother. 2021;138:111482. doi:10.1016/j.biopha.2021.111482
97. Ferreira D, Moreira JN, Rodrigues LR. New advances in exosome-based targeted drug delivery systems. Crit Rev Oncol/Hematol. 2022;172:103628. doi:10.1016/j.critrevonc.2022.103628
98. Sharma D, Maslov LN, Singh N, Jaggi AS. Remote ischemic preconditioning-induced neuroprotection in cerebral ischemia-reperfusion injury: preclinical evidence and mechanisms. Eur J Pharmacol. 2020;883:173380. doi:10.1016/j.ejphar.2020.173380
99. Che Z, Ye Z, Zhang X, et al. Mesenchymal stem/stromal cells in the pathogenesis and regenerative therapy of inflammatory bowel diseases. Front Immunol. 2022;13:952071. doi:10.3389/fimmu.2022.952071
100. Liu Y, Wang X, Chen Y, et al. Pharmacological mechanisms of traditional Chinese medicine against acute lung injury: from active ingredients to herbal formulae. Phytomedicine. 2024;135:155562. doi:10.1016/j.phymed.2024.155562
101. Aminu N, Bello I, Umar NM, Tanko N, Aminu A, Audu MM. The influence of nanoparticulate drug delivery systems in drug therapy. J Drug Delivery Sci Technol. 2020;60:101961. doi:10.1016/j.jddst.2020.101961
102. Majumder J, Minko T. Multifunctional and stimuli-responsive nanocarriers for targeted therapeutic delivery. Expert Opin Drug Delivery. 2021;18(2):205–227. doi:10.1080/17425247.2021.1828339
103. Maurya S, Srivastava R, Arfin S, et al. Exploring state-of-the-art advances in targeted nanomedicines for managing acute and chronic inflammatory lung diseases. Nanomedicine. 2022;17(30):2245–2264. doi:10.2217/nnm-2021-0437
104. Anderson CF, Grimmett ME, Domalewski CJ, Cui H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12(1):e1586. doi:10.1002/wnan.1586
105. Raviv SA, Alyan M, Egorov E, et al. Lung targeted liposomes for treating ARDS. J Control Release. 2022;346:421–433. doi:10.1016/j.jconrel.2022.03.028
106. Huang Z, Wang H, Long J, Lu Z, Chun C, Li X. Neutrophil membrane-coated therapeutic liposomes for targeted treatment in acute lung injury. Int J Pharm. 2022;624:121971. doi:10.1016/j.ijpharm.2022.121971
107. Jiang L, Guo P, Ju J, Zhu X, Wu S, Dai J. Inhalation of L-arginine-modified liposomes targeting M1 macrophages to enhance curcumin therapeutic efficacy in ALI. Eur J Pharm Biopharm. 2023;182:21–31. doi:10.1016/j.ejpb.2022.11.017
108. Su M, Hu H, Zhao X, Huang C, Yang B, Yin Z. Construction of mannose-modified polyethyleneimine-block-polycaprolactone cationic polymer micelles and its application in acute lung injury. Drug Delivery Transl Res. 2022;12(5):1080–1095. doi:10.1007/s13346-021-00976-9
109. Wan Y-H, Huan M-L, Yun C-X, et al. Construction of mPEI/pGPX4 gene therapeutic system for the effective treatment of acute lung injury. Nanotechnology. 2023;34(33):335101. doi:10.1088/1361-6528/acd198
110. Solé‐Porta A, Areny‐Balagueró A, Camprubí‐Rimblas M, et al. Efficient nebulization and pulmonary biodistribution of polymeric nanocarriers in an acute lung injury preclinical model. Small Sci;2024:2400066. doi:10.1002/smsc.202400066
111. Jin H, Luo R, Li J, et al. Inhaled platelet vesicle-decoyed biomimetic nanoparticles attenuate inflammatory lung injury. Front Pharmacol. 2022;13:1050224. doi:10.3389/fphar.2022.1050224
112. Ding Y, Lv B, Zheng J, et al. RBC-hitchhiking chitosan nanoparticles loading methylprednisolone for lung-targeting delivery. J Control Release. 2022;341:702–715. doi:10.1016/j.jconrel.2021.12.018
113. Gao Y, Dai W, Ouyang Z, Shen M, Shi X. Dendrimer-mediated intracellular delivery of fibronectin guides macrophage polarization to alleviate acute lung injury. Biomacromolecules. 2023;24(2):886–895. doi:10.1021/acs.biomac.2c01318
114. Wang L, Zhang H, Sun L, et al. Manipulation of macrophage polarization by peptide-coated gold nanoparticles and its protective effects on acute lung injury. J Nanobiotechnol. 2020;18(1):1–16. doi:10.1186/s12951-020-00593-7
115. Dos Santos Haupenthal DP, Mendes C, De bem silveira G, et al. Effects of treatment with gold nanoparticles in a model of acute pulmonary inflammation induced by lipopolysaccharide. J Biomed Mater Res Part A. 2020;108(1):103–115. doi:10.1002/jbm.a.36796
116. Wang M, Wang K, Deng G, et al. Mitochondria-modulating porous Se@ SiO2 nanoparticles provide resistance to oxidative injury in airway epithelial cells: implications for acute lung injury. Int J Nanomed. 2020;Volume 15:2287–2302. doi:10.2147/IJN.S240301
117. García-Fernández A, Sancho M, Bisbal V, et al. Targeted-lung delivery of dexamethasone using gated mesoporous silica nanoparticles. A new therapeutic approach for acute lung injury treatment. J Control Release. 2021;337:14–26. doi:10.1016/j.jconrel.2021.07.010
118. Li H, Zhu J, Wang C, et al. Paeonol loaded cyclodextrin metal-organic framework particles for treatment of acute lung injury via inhalation. Int J Pharm. 2020;587:119649. doi:10.1016/j.ijpharm.2020.119649
119. Wang Y, Li Q, Deng M, Chen K, Wang J. Self-assembled metal-organic frameworks nanocrystals synthesis and application for plumbagin drug delivery in acute lung injury therapy. Chin Chem Lett. 2022;33(1):324–327. doi:10.1016/j.cclet.2021.06.080
120. Ji H, Zhang C, Xu F, et al. Inhaled pro‐efferocytic nanozymes promote resolution of acute lung injury. Adv Sci. 2022;9(26):2201696. doi:10.1002/advs.202201696
121. Yuan R, Li Y, Han S, et al. Fe-curcumin nanozyme-mediated reactive oxygen species scavenging and anti-inflammation for acute lung injury. ACS Cent Sci. 2021;8(1):10–21. doi:10.1021/acscentsci.1c00866
122. Liu C, Fan W, Cheng WX, et al. Red emissive carbon dot superoxide dismutase nanozyme for bioimaging and ameliorating acute lung injury. Adv Funct Mater. 2023;33(19):2213856. doi:10.1002/adfm.202213856
123. Lu W, Yao J, Zhu X, Qi Y. Nanomedicines: redefining traditional medicine. Biomed Pharmacother. 2021;134:111103. doi:10.1016/j.biopha.2020.111103
124. Jogpal V, Sanduja M, Dutt R, Garg V, Tinku. Advancement of nanomedicines in chronic inflammatory disorders. Inflammopharmacology. 2022;30(2):355–368. doi:10.1007/s10787-022-00927-x
125. Liu R, Luo C, Pang Z, et al. Advances of nanoparticles as drug delivery systems for disease diagnosis and treatment. Chin Chem Lett. 2023;34(2):107518. doi:10.1016/j.cclet.2022.05.032
126. Saadat M, Manshadi MK, Mohammadi M, et al. Magnetic particle targeting for diagnosis and therapy of lung cancers. J Control Release. 2020;328:776–791. doi:10.1016/j.jconrel.2020.09.017
127. Operti MC, Bernhardt A, Grimm S, Engel A, Figdor CG, Tagit O. PLGA-based nanomedicines manufacturing: technologies overview and challenges in industrial scale-up. Int J Pharm. 2021;605:120807. doi:10.1016/j.ijpharm.2021.120807
128. Summer M, Ashraf R, Ali S, et al. Inflammatory response of nanoparticles: mechanisms, consequences, and strategies for mitigation. Chemosphere. 2024;363:142826. doi:10.1016/j.chemosphere.2024.142826
129. Mitchell MJ, Billingsley MM, Haley RM, Wechsler ME, Peppas NA, Langer R. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov. 2021;20(2):101–124. doi:10.1038/s41573-020-0090-8
130. De Leo V, Maurelli AM, Giotta L, Catucci L. Liposomes containing nanoparticles: preparation and applications. Colloids Surf B. 2022;218:112737. doi:10.1016/j.colsurfb.2022.112737
131. Farooque F, Wasi M, Mughees MM. Liposomes as drug delivery system: an updated review. J Drug Delivery Ther. 2021;11(5–S):149–158. doi:10.22270/jddt.v11i5-S.5063
132. De Leo V, Milano F, Agostiano A, Catucci L. Recent advancements in polymer/liposome assembly for drug delivery: from surface modifications to hybrid vesicles. Polymers. 2021;13(7):1027. doi:10.3390/polym13071027
133. Yan W, Leung SS, To KK. Updates on the use of liposomes for active tumor targeting in cancer therapy. Nanomedicine. 2020;15(3):303–318. doi:10.2217/nnm-2019-0308
134. Idrees H, Zaidi SZJ, Sabir A, Khan RU, Zhang X, Hassan S-U. A review of biodegradable natural polymer-based nanoparticles for drug delivery applications. Nanomaterials. 2020;10(10):1970. doi:10.3390/nano10101970
135. Maurya AK, Mishra A, Mishra N. Nanoengineered polymeric biomaterials for drug delivery system. In: Nanoengineered Biomaterials for Advanced Drug Delivery. Elsevier; 2020:109–143.
136. Leyva-Gómez G, Mendoza-Muñoz N, Del Prado-Audelo M, Ojeda-Piedra S, Zambrano-Zaragoza M, Quintanar-Guerrero D. Natural polymers in pharmaceutical nanotechnology. Nanomaterials Nanotechnol. 2021;163–215.
137. Nunes D, Andrade S, Ramalho MJ, Loureiro JA, Pereira MC. Polymeric nanoparticles-loaded hydrogels for biomedical applications: a systematic review on in vivo findings. Polymers. 2022;14(5):1010. doi:10.3390/polym14051010
138. Lockwood D. Nanozymology-connecting biology and nanotechnology, nanostructure science and technology series. Springer Nat. 2020;145:4398–4420.
139. Singh S, Rai N, Tiwari H, et al. Recent advancements in the formulation of nanomaterials-based nanozymes, their catalytic activity, and biomedical applications. ACS Appl Bio Mater. 2023;6(9):3577–3599. doi:10.1021/acsabm.3c00253
140. Mei L, Zhu S, Liu Y, Yin W, Gu Z, Zhao Y. An overview of the use of nanozymes in antibacterial applications. Chem Eng J. 2021;418:129431. doi:10.1016/j.cej.2021.129431
141. Ai Y, Hu ZN, Liang X, Sun H, Xin H, Liang Q. Recent advances in nanozymes: from matters to bioapplications. Adv Funct Mater. 2022;32(14):2110432. doi:10.1002/adfm.202110432
142. Hu Q, Zhang S, Yang Y, et al. Extracellular vesicles in the pathogenesis and treatment of acute lung injury. Military Med Res. 2022;9(1):61. doi:10.1186/s40779-022-00417-9
143. Fan Z, Jiang C, Wang Y, et al. Engineered extracellular vesicles as intelligent nanosystems for next-generation nanomedicine. Nanoscale Horiz. 2022;7(7):682–714. doi:10.1039/d2nh00070a
144. Xu M, Ji J, Jin D, et al. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): intercellular shuttles and implications in human diseases. Genes Dis. 2023;10(5):1894–1907. doi:10.1016/j.gendis.2022.03.021
145. Buratta S, Tancini B, Sagini K, et al. Lysosomal exocytosis, exosome release and secretory autophagy: the autophagic-and endo-lysosomal systems go extracellular. Int J Mol Sci. 2020;21(7):2576. doi:10.3390/ijms21072576
146. Martins TS, Vaz M, Henriques AG. A review on comparative studies addressing exosome isolation methods from body fluids. Anal Bioanal Chem. 2023;415(7):1239–1263. doi:10.1007/s00216-022-04174-5
147. Hu C, Jiang W, Lv M, et al. Potentiality of exosomal proteins as novel cancer biomarkers for liquid biopsy. Front Immunol. 2022;13:792046. doi:10.3389/fimmu.2022.792046
148. Li C, Ni Y-Q, Xu H, et al. Roles and mechanisms of exosomal non-coding RNAs in human health and diseases. Signal Transduction Targeted Ther. 2021;6(1):383. doi:10.1038/s41392-021-00779-x
149. Gurung S, Perocheau D, Touramanidou L, Baruteau J. The exosome journey: from biogenesis to uptake and intracellular signalling. Cell Commun Signaling. 2021;19(1):47. doi:10.1186/s12964-021-00730-1
150. Zhu W, Zhang Y, Wang Y. Immunotherapy strategies and prospects for acute lung injury: focus on immune cells and cytokines. Front Pharmacol. 2022;13:1103309. doi:10.3389/fphar.2022.1103309
151. Li L, Jin S, Zhang Y. Ischemic preconditioning potentiates the protective effect of mesenchymal stem cells on endotoxin-induced acute lung injury in mice through secretion of exosome. Int J Clin Exp Med. 2015;8(3):3825.
152. Chen W, Huang Y, Han J, et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol Res. 2016;64(4):831–840. doi:10.1007/s12026-016-8798-6
153. Xu N, He D, Shao Y, et al. Lung-derived exosomes in phosgene-induced acute lung injury regulate the functions of mesenchymal stem cells partially via miR-28-5p. Biomed Pharmacother. 2020;121:109603. doi:10.1016/j.biopha.2019.109603
154. Liu F, Peng W, Chen J, et al. Exosomes derived from alveolar epithelial cells promote alveolar macrophage activation mediated by miR-92a-3p in sepsis-induced acute lung injury. Front Cell Infect Microbiol. 2021;11:646546. doi:10.3389/fcimb.2021.646546
155. Wang X, Liu D, Zhang X, Yang L, Xia Z, Zhang Q. Exosomes from adipose-derived mesenchymal stem cells alleviate sepsis-induced lung injury in mice by inhibiting the secretion of IL-27 in macrophages. Cell Death Discovery. 2022;8(1):18. doi:10.1038/s41420-021-00785-6
156. Liu J, Chen T, Lei P, Tang X, Huang P. Exosomes released by bone marrow mesenchymal stem cells attenuate lung injury induced by intestinal ischemia reperfusion via the TLR4/NF-κB pathway. Int J Med Sci. 2019;16(9):1238. doi:10.7150/ijms.35369
157. Li C, Li X, Shi Z, et al. Exosomes from LPS-preconditioned bone marrow MSCs accelerated peripheral nerve regeneration via M2 macrophage polarization: involvement of TSG-6/NF-κB/NLRP3 signaling pathway. Exp Neurol. 2022;356:114139. doi:10.1016/j.expneurol.2022.114139
158. Wang Y, Wang L, Ma S, Cheng L, Yu G. Repair and regeneration of the alveolar epithelium in lung injury. FASEB J. 2024;38(8):e23612. doi:10.1096/fj.202400088R
159. Wei Li J, Wei L, Han Z, Chen Z. Mesenchymal stromal cells-derived exosomes alleviate ischemia/reperfusion injury in mouse lung by transporting anti-apoptotic miR-21-5p. Eur J Pharmacol. 2019;852:68–76. doi:10.1016/j.ejphar.2019.01.022
160. Wu X, Jiang Y, Li R, et al. Ficolin B secreted by alveolar macrophage exosomes exacerbates bleomycin-induced lung injury via ferroptosis through the cGAS-STING signaling pathway. Cell Death Dis. 2023;14(8):577. doi:10.1038/s41419-023-06104-4
161. Mizuta Y, Akahoshi T, Guo J, et al. Exosomes from adipose tissue-derived mesenchymal stem cells ameliorate histone-induced acute lung injury by activating the PI3K/Akt pathway in endothelial cells. Stem Cell Res Ther. 2020;11(1):1–12. doi:10.1186/s13287-020-02015-9
162. Liang B, Liang J-M, Ding J-N, Xu J, Xu J-G, Chai Y-M. Dimethyloxaloylglycine-stimulated human bone marrow mesenchymal stem cell-derived exosomes enhance bone regeneration through angiogenesis by targeting the AKT/mTOR pathway. Stem Cell Res Ther. 2019;10(1):1–11. doi:10.1186/s13287-019-1410-y
163. Zhang Y, Hao Z, Wang P, et al. Exosomes from human umbilical cord mesenchymal stem cells enhance fracture healing through HIF‐1α‐mediated promotion of angiogenesis in a rat model of stabilized fracture. Cell Proliferation. 2019;52(2):e12570. doi:10.1111/cpr.12570
164. Zhou Y, Li P, Goodwin AJ, et al. Exosomes from endothelial progenitor cells improve outcomes of the lipopolysaccharide-induced acute lung injury. Critical Care. 2019;23(1):1–12. doi:10.1186/s13054-019-2339-3
165. Feng Z, Jing Z, Li Q, et al. Exosomal STIMATE derived from type II alveolar epithelial cells controls metabolic reprogramming of tissue-resident alveolar macrophages. Theranostics. 2023;13(3):991. doi:10.7150/thno.82552